Exacerbated lung inflammation following secondary RSV exposure is CD4+ T cell-dependent and is not mitigated in infant BALB/c mice born to PreF-vaccinated dams
- PMID: 37646035
- PMCID: PMC10461110
- DOI: 10.3389/fimmu.2023.1206026
Exacerbated lung inflammation following secondary RSV exposure is CD4+ T cell-dependent and is not mitigated in infant BALB/c mice born to PreF-vaccinated dams
Abstract
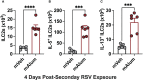
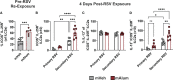
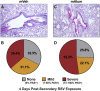
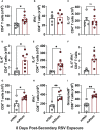
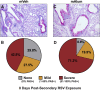
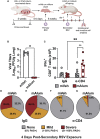
Respiratory syncytial virus (RSV) is the leading cause of childhood hospitalizations due to bronchiolitis in children under 5 years of age. Moreover, severe RSV disease requiring hospitalization is associated with the subsequent development of wheezing and asthma. Due to the young age in which viral protection is needed and risk of vaccine enhanced disease following direct infant vaccination, current approaches aim to protect young children through maternal immunization strategies that boost neutralizing maternal antibody (matAb) levels. However, there is a scarcity of studies investigating the influence of maternal immunization on secondary immune responses to RSV in the offspring or whether the subsequent development of wheezing and asthma is mitigated. Toward this goal, our lab developed a murine model of maternal RSV vaccination and repeat RSV exposure to evaluate the changes in immune response and development of exacerbated lung inflammation on secondary RSV exposure in mice born to immunized dams. Despite complete protection following primary RSV exposure, offspring born to pre-fusion F (PreF)-vaccinated dams had exaggerated secondary ILC2 and Th2 responses, characterized by enhanced production of IL-4, IL-5, and IL-13. These enhanced type 2 cellular responses were associated with exaggerated airway eosinophilia and mucus hyperproduction upon re-exposure to RSV. Importantly, depletion of CD4+ T cells led to complete amelioration of the observed type 2 pathology on secondary RSV exposure. These unanticipated results highlight the need for additional studies that look beyond primary protection to better understand how maternal immunization shapes subsequent immune responses to repeat RSV exposure.
Keywords: Respiratory syncytial virus; T cell-mediated pathology; maternal immunization; secondary RSV exposure; type 2 inflammation.
Copyright © 2023 Kosanovich, Eichinger, Lipp, Gidwani, Brahmbhatt, Yondola, Perkins and Empey.
Conflict of interest statement
SG, DB, and MY are employed by Calder Biosciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet (2017) 390(10098):946–58. doi: 10.1016/S0140-6736(17)30938-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

