Safety of cangrelor and transition to oral P2Y12 inhibitors in patients undergoing percutaneous coronary intervention: the ARCANGELO study
- PMID: 37646045
- PMCID: PMC10462400
- DOI: 10.1093/ehjopen/oead076
Safety of cangrelor and transition to oral P2Y12 inhibitors in patients undergoing percutaneous coronary intervention: the ARCANGELO study
Abstract
Aims: Cangrelor is the only intravenous P2Y12 inhibitor available. Safety, efficacy, and transitioning from cangrelor to oral P2Y12 inhibitors were recorded in patients with acute coronary syndrome (ACS). The ARCANGELO study aims to assess the safety of cangrelor on bleeding and the effects of the transition to oral P2Y12 inhibitors in a real-world setting according to the European Medical Agency's requirement.
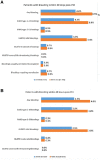
Methods and results: Adult patients with ACS undergoing percutaneous coronary intervention (PCI) receiving cangrelor were included in the study. Patients were followed for 30 days. Incidence of bleeding events, major adverse cardiac events, and transition strategy to oral P2Y12 were recorded. Among 1004 ACS patients undergoing PCI, 995 (99.1%) were eligible for the analysis; 597 (60.0%) of them had ST-segment elevation myocardial infarction. A total of 925 (93.1%) patients underwent PCI by radial catheter access, and 972 (97.2%) received drug-eluting stents. All eligible patients received bolus and cangrelor infusion between 2 and 4 h in 95% of the cases. A total of 730 patients (73.4%) received ticagrelor, 127 (12.8%) prasugrel, and 138 (13.9%) clopidogrel as transition therapy. Bleeding, according to Bleeding Academic Research Consortium (BARC) criteria, within 30 days post-PCI occurred in 5.2% of patients (95% confidence interval: 3.9-6.8%); 0.5% experienced a moderate (BARC 3), and all others mild (BARC 1-2) bleeding events. Major adverse cardiac events occurred in 14 (1.4%) patients, principally all-cause mortality (n = 6 patients) and myocardial infarction (n = 7 patients).
Conclusion: The use of cangrelor in ACS patients undergoing PCI and the transition strategy to P2Y12 inhibitors are confirmed as safe and effective in daily practice.
Keywords: Acute coronary syndrome; Bleeding; Cangrelor; P2Y12 receptor inhibitor; Percutaneous coronary intervention.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: All the activities were funded by Chiesi Farmaceutici S.p.A. (Parma, Italy).
Figures



References
-
- Leonardi S, Mahaffey KW, White HD, Gibson CM, Stone GW, Steg GW, Hamm CW, Price MJ, Todd M, Dietrich M, Gallup D, Liu T, Skerjanec S, Harrington RA, Bhatt DL. Rationale and design of the cangrelor versus standard therapy to achieve optimal management of platelet inhibition PHOENIX trial. Am Heart J 2012;163:768–776.e2. - PubMed
-
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel. Clinical implications, management, and future perspectives. J Am Coll Cardiol 2007;49:1505–1516. - PubMed
-
- de Luca L, Leonardi S, Cavallini C, Lucci D, Musumeci G, Caporale R, Abrignani MG, Lupi A, Rakar S, Gulizia MM, Bovenzi FM, De Servi S. Contemporary antithrombotic strategies in patients with acute coronary syndrome admitted to cardiac care units in Italy: the EYESHOT Study. Eur Heart J Acute Cardiovasc Care 2015;4:441–452. - PubMed
-
- Gragnano F, Mehran R, Branca M, Franzone A, Baber U, Jang Y, Kimura T, Hahn JY, Zhao Q, Windecker S, Gibson CM, Kim BK, Watanabe H, Song YB, Zhu Y, Vranckx P, Mehta S, Hong SJ, Ando K, Gwon HC, Calabrò P, Serruys PW, Dangas GD, McFadden EP, Angiolillo DJ, Heg D, Valgimigli M; Single Versus Dual Antiplatelet Therapy (Sidney-2) Collaboration . P2y12 inhibitor monotherapy or dual antiplatelet therapy after complex percutaneous coronary interventions. J Am Coll Cardiol 2023;81:537–552. - PubMed
-
- Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, Price MJ, Leonardi S, Gallup D, Bramucci E, Radke PW, Widimský P, Tousek F, Tauth J, Spriggs D, McLaurin BT, Angiolillo DJ, Généreux P, Liu T, Prats J, Todd M, Skerjanec S, White HD, Harrington RA; CHAMPION PHOENIX Investigators . Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 2013;368:1303–1313. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

