Highly Reduced Ruthenium Carbide Carbonyl Clusters: Synthesis, Molecular Structure, Reactivity, Electrochemistry, and Computational Investigation of [Ru6C(CO)15]4
- PMID: 37646082
- PMCID: PMC10498495
- DOI: 10.1021/acs.inorgchem.3c01711
Highly Reduced Ruthenium Carbide Carbonyl Clusters: Synthesis, Molecular Structure, Reactivity, Electrochemistry, and Computational Investigation of [Ru6C(CO)15]4
Abstract
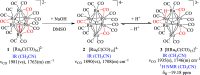
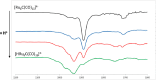
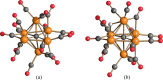
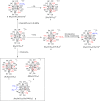
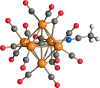
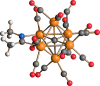
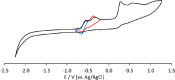
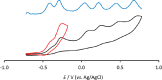
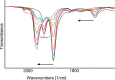
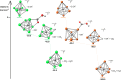
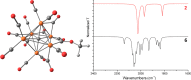
The reaction of [Ru6C(CO)16]2- (1) with NaOH in DMSO resulted in the formation of a highly reduced [Ru6C(CO)15]4- (2), which was readily protonated by acids, such as HBF4·Et2O, to [HRu6C(CO)15]3- (3). Oxidation of 2 with [Cp2Fe][PF6] or [C7H7][BF4] in CH3CN resulted in [Ru6C(CO)15(CH3CN)]2- (5), which was quantitatively converted into 1 after exposure to CO atmosphere. The reaction of 2 with a mild methylating agent such as CH3,I afforded the purported [Ru6C(CO)14(COCH3)]3- (6). By employing a stronger reagent, that is, CF3SO3CH3, a mixture of [HRu6C(CO)16]- (4), [H3Ru6C(CO)15]- (7), and [Ru6C(CO)15(CH3CNCH3)]- (8) was obtained. The molecular structures of 2-5, 7, and 8 were determined by single-crystal X-ray diffraction as their [NEt4]4[2]·CH3CN, [NEt4]3[3], [NEt4][4], [NEt4]2[5], [NEt4][7], and [NEt4][8]·solv salts. The carbyne-carbide cluster 6 was partially characterized by IR spectroscopy and ESI-MS, and its structure was computationally predicted using DFT methods. The redox behavior of 2 and 3 was investigated by electrochemical and IR spectroelectrochemical methods. Computational studies were performed in order to unravel structural and thermodynamic aspects of these octahedral Ru-carbide carbonyl clusters displaying miscellaneous ligands and charges in comparison with related iron derivatives.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
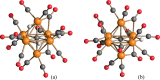

Similar articles
-
Chemical and Electrochemical Investigation of the Oxidation of a Highly Reduced Fe6C Iron Carbide Carbonyl Cluster: A Synthetic Route to Heteroleptic Fe6C and Fe5C Clusters.Inorg Chem. 2025 May 19;64(19):9744-9757. doi: 10.1021/acs.inorgchem.5c01014. Epub 2025 May 6. Inorg Chem. 2025. PMID: 40327361 Free PMC article.
-
The redox chemistry of [Co6C(CO)15](2-): a synthetic route to new co-carbide carbonyl clusters.Inorg Chem. 2014 Apr 7;53(7):3818-31. doi: 10.1021/ic500161e. Epub 2014 Mar 21. Inorg Chem. 2014. PMID: 24654982
-
Structures, Interconversions, and Spectroscopy of Iron Carbonyl Clusters with an Interstitial Carbide: Localized Metal Center Reduction by Overall Cluster Oxidation.Inorg Chem. 2017 May 15;56(10):5998-6012. doi: 10.1021/acs.inorgchem.7b00741. Epub 2017 Apr 25. Inorg Chem. 2017. PMID: 28441025 Free PMC article.
-
Molecular Nickel Phosphide Carbonyl Nanoclusters: Synthesis, Structure, and Electrochemistry of [Ni11P(CO)18]3- and [H6-nNi31P4(CO)39]n- (n = 4 and 5).Inorg Chem. 2018 Feb 5;57(3):1136-1147. doi: 10.1021/acs.inorgchem.7b02598. Epub 2018 Jan 5. Inorg Chem. 2018. PMID: 29303559
-
Metal Segregation in Bimetallic CoPd Carbide Carbonyl Clusters: Synthesis, Structure, Reactivity and Electrochemistry of [H6-n Co20 Pd16 C4 (CO)48 ]n- (n=3-6).Chempluschem. 2013 Dec;78(12):1456-1465. doi: 10.1002/cplu.201300268. Epub 2013 Oct 2. Chempluschem. 2013. PMID: 31986665
Cited by
-
Preparation of Ru-Based Systems Through Metal Carbonyl Cluster Decomposition for the Base-Free 5-Hydroxymethylfurfural (HMF) Oxidation.Molecules. 2025 May 10;30(10):2120. doi: 10.3390/molecules30102120. Molecules. 2025. PMID: 40430293 Free PMC article.
-
Chemical and Electrochemical Investigation of the Oxidation of a Highly Reduced Fe6C Iron Carbide Carbonyl Cluster: A Synthetic Route to Heteroleptic Fe6C and Fe5C Clusters.Inorg Chem. 2025 May 19;64(19):9744-9757. doi: 10.1021/acs.inorgchem.5c01014. Epub 2025 May 6. Inorg Chem. 2025. PMID: 40327361 Free PMC article.
References
-
- Johnson B. F. G.; Jonston R. D.; Lewis J. Ruthenium carbonyl carbide compounds. Chem. Commun. 1967, 1057.10.1039/c1967001057a. - DOI
-
- Sirigu A.; Bianchi M.; Benedetti E. The Crystal Structure of Ru6C(CO)17. Chem. Commun. 1969, 596.10.1039/c2969000596a. - DOI
-
- Eady C. R.; Johnson B. F. G.; Lewis J. The Chemistry of Polynuclear Compounds. Part XXVI. Products of the Pyrolysis of Dodecacarbonyl-triangulo-triruthenium and -triosmium. J. Chem. Soc., Dalton Trans. 1975, 2606–2611. 10.1039/dt9750002606. - DOI
-
- Cesari C.; Femoni C.; Carmela Iapalucci M.; Zacchini S. Molecular Fe, Co and Ni carbide carbonyl clusters and nanoclusters. Inorg. Chim. Acta 2023, 544, 121235.10.1016/j.ica.2022.121235. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

