Mammalian display to secretion switchable libraries for antibody preselection and high throughput functional screening
- PMID: 37646089
- PMCID: PMC10469430
- DOI: 10.1080/19420862.2023.2251190
Mammalian display to secretion switchable libraries for antibody preselection and high throughput functional screening
Abstract
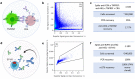
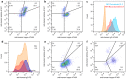
Recently, there has been a co-evolution of mammalian libraries and diverse microfluidic approaches for therapeutic antibody hit discovery. Mammalian libraries enable the preservation of full immune repertoires, produce hit candidates in final format and facilitate broad combinatorial bispecific antibody screening, while several available microfluidic methodologies offer opportunities for rapid high-content screens. Here, we report proof-of-concept studies exploring the potential of combining microfluidic technologies with mammalian libraries for antibody discovery. First, antibody secretion, target co-expression and integration of appropriate reporter cell lines enabled the selection of in-trans acting agonistic bispecific antibodies. Second, a functional screen for internalization was established and comparison of autocrine versus co-encapsulation setups highlighted the advantages of an autocrine one cell approach. Third, synchronization of antibody-secreting cells prior to microfluidic screens reduced assay variability. Furthermore, a display to secretion switchable system was developed and applied for pre-enrichment of antibody clones with high manufacturability in conjunction with subsequent screening for functional properties. These case studies demonstrate the system's feasibility and may serve as basis for further development of integrated workflows combining manufacturability sorting and functional screens for the identification of optimal therapeutic antibody candidates.
Keywords: Antibodies; display secretion switch; function first screen; mammalian libraries; microfluidics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Gérard A, Woolfe A, Mottet G, Reichen M, Castrillon C, Menrath V, Ellouze S, Poitou A, Doineau R, Briseno-Roa L, et al. High-throughput single-cell activity-based screening and sequencing of antibodies using droplet microfluidics. Nat Biotechnol. 2020;38(6):715–10. doi:10.1038/s41587-020-0466-7. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
