Proteomic Atlas of Atherosclerosis: The Contribution of Proteoglycans to Sex Differences, Plaque Phenotypes, and Outcomes
- PMID: 37646165
- PMCID: PMC10498884
- DOI: 10.1161/CIRCRESAHA.123.322590
Proteomic Atlas of Atherosclerosis: The Contribution of Proteoglycans to Sex Differences, Plaque Phenotypes, and Outcomes
Abstract
Background: Using proteomics, we aimed to reveal molecular types of human atherosclerotic lesions and study their associations with histology, imaging, and cardiovascular outcomes.
Methods: Two hundred nineteen carotid endarterectomy samples were procured from 120 patients. A sequential protein extraction protocol was employed in conjunction with multiplexed, discovery proteomics. To focus on extracellular proteins, parallel reaction monitoring was employed for targeted proteomics. Proteomic signatures were integrated with bulk, single-cell, and spatial RNA-sequencing data, and validated in 200 patients from the Athero-Express Biobank study.
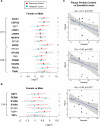
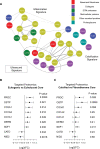
Results: This extensive proteomics analysis identified plaque inflammation and calcification signatures, which were inversely correlated and validated using targeted proteomics. The inflammation signature was characterized by the presence of neutrophil-derived proteins, such as S100A8/9 (calprotectin) and myeloperoxidase, whereas the calcification signature included fetuin-A, osteopontin, and gamma-carboxylated proteins. The proteomics data also revealed sex differences in atherosclerosis, with large-aggregating proteoglycans versican and aggrecan being more abundant in females and exhibiting an inverse correlation with estradiol levels. The integration of RNA-sequencing data attributed the inflammation signature predominantly to neutrophils and macrophages, and the calcification and sex signatures to smooth muscle cells, except for certain plasma proteins that were not expressed but retained in plaques, such as fetuin-A. Dimensionality reduction and machine learning techniques were applied to identify 4 distinct plaque phenotypes based on proteomics data. A protein signature of 4 key proteins (calponin, protein C, serpin H1, and versican) predicted future cardiovascular mortality with an area under the curve of 75% and 67.5% in the discovery and validation cohort, respectively, surpassing the prognostic performance of imaging and histology.
Conclusions: Plaque proteomics redefined clinically relevant patient groups with distinct outcomes, identifying subgroups of male and female patients with elevated risk of future cardiovascular events.
Keywords: atherosclerosis; inflammation; machine learning; neutrophils; proteoglycans; proteomics; smooth muscle cells.
Conflict of interest statement
Figures






References
-
- Holm Nielsen S, Jonasson L, Kalogeropoulos K, Karsdal MA, Reese-Petersen AL, auf dem Keller U, Genovese F, Nilsson J, Goncalves I. Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. J Inter Med. 2020;287:493–513. doi: 10.1111/JOIM.13034 - PubMed
-
- Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–1373. doi: 10.1161/01.cir.0000028591.44554.f9 - PubMed
-
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

