Pharmacology and Therapeutic Potential of Benzothiazole Analogues for Cocaine Use Disorder
- PMID: 37646374
- PMCID: PMC10510399
- DOI: 10.1021/acs.jmedchem.3c00734
Pharmacology and Therapeutic Potential of Benzothiazole Analogues for Cocaine Use Disorder
Abstract
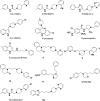
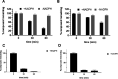
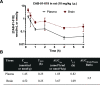
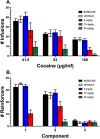
Pharmacological targeting of the dopamine D4 receptor (D4R)─expressed in brain regions that control cognition, attention, and decision-making─could be useful for several neuropsychiatric disorders including substance use disorders (SUDs). This study focused on the synthesis and evaluation of a novel series of benzothiazole analogues designed to target D4R. We identified several compounds with high D4R binding affinity (Ki ≤ 6.9 nM) and >91-fold selectivity over other D2-like receptors (D2R, D3R) with diverse partial agonist and antagonist profiles. Novel analogue 16f is a potent low-efficacy D4R partial agonist, metabolically stable in rat and human liver microsomes, and has excellent brain penetration in rats (AUCbrain/plasma > 3). 16f (5-30 mg/kg, i.p.) dose-dependently decreased iv cocaine self-administration in rats, consistent with previous results produced by D4R-selective antagonists. Off-target antagonism of 5-HT2A or 5-HT2B may also contribute to these effects. Results with 16f support further efforts to target D4R in SUD treatment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
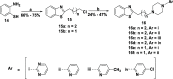


References
-
- Keck T. M.; Free R. B.; Day M. M.; Brown S. L.; Maddaluna M. S.; Fountain G.; Cooper C.; Fallon B.; Holmes M.; Stang C. T.; Burkhardt R.; Bonifazi A.; Ellenberger M. P.; Newman A. H.; Sibley D. R.; Wu C.; Boateng C. A. Dopamine D4 Receptor-Selective Compounds Reveal Structure-Activity Relationships that Engender Agonist Efficacy. J. Med. Chem. 2019, 62, 3722–3740. 10.1021/acs.jmedchem.9b00231. - DOI - PMC - PubMed
-
- Tomlinson A.; Grayson B.; Marsh S.; Hayward A.; Marshall K. M.; Neill J. C. Putative therapeutic targets for symptom subtypes of adult ADHD: D4 receptor agonism and COMT inhibition improve attention and response inhibition in a novel translational animal model. Eur. Neuropsychopharmacol. 2015, 25, 454–467. 10.1016/j.euroneuro.2014.11.016. - DOI - PubMed
-
- Ferre S.; Belcher A. M.; Bonaventura J.; Quiroz C.; Sanchez-Soto M.; Casado-Anguera V.; Cai N. S.; Moreno E.; Boateng C. A.; Keck T. M.; Floran B.; Earley C. J.; Ciruela F.; Casado V.; Rubinstein M.; Volkow N. D. Functional and pharmacological role of the dopamine D4 receptor and its polymorphic variants. Front. Endocrinol. (Lausanne) 2022, 13, 1014678. 10.3389/fendo.2022.1014678. - DOI - PMC - PubMed
-
- Botticelli L.; Micioni Di Bonaventura E.; Del Bello F.; Giorgioni G.; Piergentili A.; Romano A.; Quaglia W.; Cifani C.; Micioni Di Bonaventura M. V. Underlying Susceptibility to Eating Disorders and Drug Abuse: Genetic and Pharmacological Aspects of Dopamine D4 Receptors. Nutrients 2020, 12, 2288. 10.3390/nu12082288. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Medical

