The mature N-termini of Plasmodium effector proteins confer specificity of export
- PMID: 37646514
- PMCID: PMC10653839
- DOI: 10.1128/mbio.01215-23
The mature N-termini of Plasmodium effector proteins confer specificity of export
Abstract
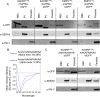
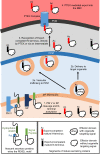
Malaria parasites export hundreds of proteins to the cytoplasm of the host red blood cells for their survival. A five amino acid sequence, called the PEXEL motif, is conserved among many exported proteins and is thought to be a signal for export. However, the motif is cleaved inside the endoplasmic reticulum of the parasite, and mature proteins starting from the fourth PEXEL residue travel to the parasite periphery for export. We showed that the PEXEL motif is dispensable for export as long as identical mature proteins can be efficiently produced via alternative means in the ER. We also showed that the exported and non-exported proteins are differentiated at the parasite periphery based on their mature N-termini; however, any discernible export signal within that region remained cryptic. Our study resolves a longstanding paradox in PEXEL protein trafficking.
Keywords: PEXEL; PTEX; Plasmodium falciparum; malaria; plasmepsin; protein export; signal peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Marapana DS, Dagley LF, Sandow JJ, Nebl T, Triglia T, Pasternak M, Dickerman BK, Crabb BS, Gilson PR, Webb AI, Boddey JA, Cowman AF. 2018. Plasmepsin V cleaves malaria effector proteins in a distinct endoplasmic reticulum translocation interactome for export to the erythrocyte. Nat Microbiol 3:1010–1022. doi:10.1038/s41564-018-0219-2 - DOI - PubMed
-
- Boddey JA, O’Neill MT, Lopaticki S, Carvalho TG, Hodder AN, Nebl T, Wawra S, van West P, Ebrahimzadeh Z, Richard D, Flemming S, Spielmann T, Przyborski J, Babon JJ, Cowman AF. 2016. Export of malaria proteins requires co-translational processing of the PEXEL motif independent of phosphatidylinositol-3-phosphate binding. Nat Commun 7:10470. doi:10.1038/ncomms10470 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

