Real-World Glycemic Outcomes with Early Omnipod 5 Use in Youth with Type 1 Diabetes
- PMID: 37646634
- PMCID: PMC10771875
- DOI: 10.1089/dia.2023.0337
Real-World Glycemic Outcomes with Early Omnipod 5 Use in Youth with Type 1 Diabetes
Abstract
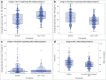
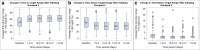
Background: Pivotal trials of diabetes technologies have demonstrated glycemic improvements; however, these trials include patients of limited diversity and ranges of glycemic control. We assessed changes in glycemic control during the first 90 days of Omnipod 5 use in a real-world cohort of youth with type 1 diabetes (T1D). Methods: Youth 2-21 years with T1D initiating Omnipod 5 at two pediatric academic centers were included. Fourteen days of baseline (BL) continuous glucose monitoring (CGM) data were compared against data from the first 90 days of Omnipod 5 use. Outcome measures included changes in time in range (TIR), hemoglobin A1c (HbA1c), and CGM and insulin pump metrics based on the duration of Omnipod 5 use. Results: Among 195 youth (78.9% non-Hispanic White, 15.4% publicly insured, age 11.7 years, T1D duration 3.3 years) TIR increased 11%-points, from 49% to 61% (P < 0.001), and HbA1c decreased 0.5%-points, from 7.5% to 6.9% (P < 0.001). TIR improved within the first 9 days of Omnipod 5 use (p < 0.001) and did not change significantly thereafter (P = 0.1) despite decreases in user-initiated boluses (5.1 vs. 5.0, P = 0.01) and carbohydrate entries (4.2 vs. 4.1, P = 0.005) from days 1-9 to days 1-90. TIR improved 15%-points among youth with BL TIR <60% compared to a 5%-point increase for youth with BL TIR ≥60% (P < 0.001). Conclusions: Glycemic control improved within 9 days of Omnipod 5 initiation in this real-world cohort, and improvements were sustained over the first 90 days of use despite concomitant decreases in user-initiated boluses. These improvements were comparable to those observed in the pivotal trial.
Keywords: Automated insulin delivery; Barriers; Glycemic control; Inequities; Minority youth; Type 1 diabetes.
Conflict of interest statement
B.E.M. is supported by the National Institutes of Health (PI: B.E.M., NIH: K23DK129827) and has received investigator-initiated research support from Tandem Diabetes Care, Inc., the Cystic Fibrosis Foundation, industry sponsored research support from Medtronic, and research supplies from Dexcom, Inc., and Digostics. R.M.W. is supported by the National Institutes of Health (1R01DK134955 and 1R01EY033233). R.M.W. is the site PI of a Novo Nordisk sponsored clinical trial.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

