Loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma: long-term efficacy and safety from the phase II LOTIS-2 study
- PMID: 37646659
- PMCID: PMC10985439
- DOI: 10.3324/haematol.2023.283459
Loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma: long-term efficacy and safety from the phase II LOTIS-2 study
Abstract
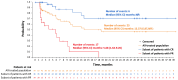
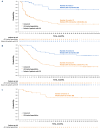
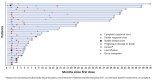
Therapies that demonstrate durable, long-term responses with manageable safety and tolerability are needed for patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). Loncastuximab tesirine (loncastuximab tesirine-lpyl [Lonca]), an anti-CD19 antibody conjugated to a potent pyrrolobenzodiazepine dimer, demonstrated single-agent antitumor activity in the pivotal phase II LOTIS-2 study in heavily pretreated patients with R/R DLBCL. Here we present updated efficacy and safety analyses from LOTIS-2, performed for all patients and in subsets of patients with a complete response (CR), including patients with CR who were event-free (no progressive disease or death) for ≥1 year and ≥2 years from cycle 1, day 1 of treatment. Lonca was administered every 3 weeks (0.15 mg/kg for 2 cycles; 0.075 mg/kg for subsequent cycles). As of the final data cutoff (September 15, 2022; median follow-up: 7.8 months [range, 0.3-42.6]), 70 of 145 (48.3%) patients achieved an overall response. Thirty-six (24.8%) patients achieved CR, of which 16 (44%) and 11 (31%) were event-free for ≥1 year and ≥2 years, respectively. In the all-treated population, the median overall survival was 9.5 months; the median progression-free survival was 4.9 months. Among patients with CR, median overall survival and progression-free survival were not reached, with 24-month overall and progression-free survival rates of 68.2% (95% CI: 50.0-81.0) and 72.5% (95% CI: 48.2-86.8), respectively. No new safety concerns were detected. With additional follow-up, Lonca continued to demonstrate durable, long-term responses with manageable safety and tolerability in patients with CR (clinicaltrials gov. Identifier: NCT03589469).
Figures
Comment in
-
More than a lonca-shot: beating the odds in relapsed/ refractory diffuse large B-cell lymphoma.Haematologica. 2024 Apr 1;109(4):1022-1024. doi: 10.3324/haematol.2023.284056. Haematologica. 2024. PMID: 37794796 Free PMC article. No abstract available.
References
-
- Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood. 2022;139(18):2737-2746. - PubMed
-
- Kansagra A, Farnia S, Majhail N. Expanding access to chimeric antigen receptor T-cell therapies: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2020;40:1-8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

