Lisocabtagene maraleucel for second-line relapsed or refractory large B-cell lymphoma: patient-reported outcomes from the PILOT study
- PMID: 37646670
- PMCID: PMC10905070
- DOI: 10.3324/haematol.2023.283162
Lisocabtagene maraleucel for second-line relapsed or refractory large B-cell lymphoma: patient-reported outcomes from the PILOT study
Abstract
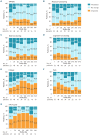
In the single-arm, open-label, multicenter, phase II PILOT study, second-line treatment with the chimeric antigen receptor (CAR) T-cell therapy lisocabtagene maraleucel (liso-cel) in patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) for whom hematopoietic stem cell transplantation (HSCT) was not intended resulted in high response rates, durable responses, and a safety profile consistent with previous reports. Here, we analyzed changes in health-related quality of life (HRQOL) in patients who received liso-cel in PILOT. Patients received liso-cel, an autologous, CD19-directed, 4-1BB CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells, for a total target dose of 100×10⁶ CAR+ T cells. HRQOL, a secondary endpoint of PILOT, was assessed as prespecified using three patient-reported outcome instruments (EORTC QLQ-C30; FACT-LymS; EQ-5D-5L). Evaluable datasets for the EORTC QLQ-C30, FACT-LymS, and EQ-5D-5L health utility index, and visual analog scale (EQ-VAS) included 56 (92%), 49 (80%), 55 (90%), and 54 (89%) patients, respectively. Clinically meaningful improvement was achieved across most post-treatment visits for EORTC QLQ-C30 fatigue and FACT-LymS. Overall mean changes from baseline through day 545 showed significant improvements in EORTC QLQ-C30 fatigue, pain, and appetite loss, FACT-LymS, and EQ VAS. In within-patient analyses, clinically meaningful improvements or maintenance in scores were observed in most patients at days 90, 180, 270, and 365. HRQOL was maintained or improved in patients who received liso-cel as second-line therapy in PILOT. These findings support liso-cel as a preferred second-line treatment in patients with R/R LBCL not intended for HSCT (clinicaltrials gov. Identifier: NCT03483103).
Figures

References
-
- Kamdar M, Solomon SR, Arnason J, et al. .; for the TRANSFORM investigators. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294-2308. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. .; for all ZUMA-7 investigators and contributing Kite members. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. - PubMed
-
- Bishop MR, Dickinson M, Purtill D, et al. . Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629-639. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

