Analysis of inflammatory markers and tau deposits in an autopsy series of nine patients with anti-IgLON5 disease
- PMID: 37646790
- PMCID: PMC10499680
- DOI: 10.1007/s00401-023-02625-6
Analysis of inflammatory markers and tau deposits in an autopsy series of nine patients with anti-IgLON5 disease
Abstract
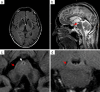
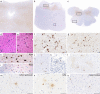
Anti-IgLON5 disease is a rare neurological, probably autoimmune, disorder associated in many cases with a specific tauopathy. Only a few post-mortem neuropathological studies have been reported so far. Little is known about the pathogenic mechanisms that result in neurodegeneration. We investigated the neuropathology of anti-IgLON5 disease and characterized cellular and humoral inflammation. We included nine cases (six of them previously published). Median age of patients was 71 years (53-82 years), the median disease duration was 6 years (0.5-13 years), and the female to male ratio was 5:4. Six cases with a median disease duration of 9 years presented a prominent tauopathy. Five of them had a classical anti-IgLON5-related brainstem tauopathy and another presented a prominent neuronal and glial 4-repeat tauopathy, consistent with progressive supranuclear palsy (PSP). Three cases with short disease duration (median 1.25 years) only showed a primary age-related neurofibrillary pathology. Inflammatory infiltrates of T and B cells were mild to moderate and did not significantly differ between anti-IgLON5 disease cases with or without tauopathy. In contrast, we found an extensive neuropil deposition of IgG4 in the tegmentum of the brainstem, olivary nucleus, and cerebellar cortex that was most prominent in two patients with short disease duration without the typical IgLON5-related tauopathy. The IgG4 deposits were particularly prominent in the cerebellar cortex and in these regions accompanied by mild IgG1 deposits. Activated complement deposition (C9neo) was absent. Our study indicates that IgLON5-related tau pathology occurs in later disease stages and may also present a PSP-phenotype with exclusively 4-repeat neuronal and glial tau pathology. The prominent deposition of anti-IgLON5 IgG4 at predilection sites for tau pathology suggests that anti-IgLON5 antibodies precede the tau pathology. Early start of immunotherapy might prevent irreversible neuronal damage and progression of the disease, at least in a subgroup of patients.
© 2023. The Author(s).
Conflict of interest statement
E. Berger-Sieczkowski, V. Endmayr, C. Haider, G. Ricken, P. Jauk, S. Macher, Schnider, E. Bradley-Zechmeister, R. Reinecke, Reinecke Raphael, G. Kasprian, C. Weber, M. Bergmann, C. Gaig, L. Sabater, and E. Gelpi report no relevant disclosures. I. Koneczny has participated in meetings sponsored by Alexion. I. Milenkovic has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from: UCB, Sanofi-Genzyme, BioMarin and Takeda. A. Heidbreder reports speaker honoraria for UCB, Bioprojet, Idorsia, Medice, Jazz Pharmaceuticals. B. Högl reports speaker honoraria Jazz and Abbvie and advisor feed from Lundbeck. S. Mariotto reports speaker honoraria from Biogen, Sanofy, and Novartis. T. Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Biogen, Biologix, Bionorica, BMS/Celgene, Eisei, Janssen-Cilag, MedDay, Merck, Novartis, Roche, Sandoz, Sanofi-Genzyme, Teva, UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Bayer, Biogen, BMS/Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, BMS/Celgene, Merck, Novartis, Roche, Sanofi-Aventis, Teva. R. Höftberger reports speaker honoraria from Novartis and Biogen. The Medical University of Vienna (Austria; employer of Dr. Höftberger) receives payment for antibody assays and for antibody validation experiments organized by Euroimmun (Lübeck, Germany). Dr. F. Graus holds a patent licensed to Euroimmun for the use of IgLON5 in an autoantibody test, for which he receives royalties, and receives honoraria from MedLink Neurology for his role as associate editor. W. Pirker attended advisory boards by Stada, AbbVie Pharma, Merz and Bial. He received travel grants and educational support from Boehringer Ingelheim, AOP Pharma as well as lecturing honoraria from GE, UCB, Bial, Stada, AOP Orphan, AbbVie, Medtronic and Merz Pharma.
Figures



References
-
- Altendorfer B, Unger MS, Poupardin R, Hoog A, Asslaber D, Gratz IK, et al. Transcriptomic profiling identifies CD8(+) t cells in the brain of aged and Alzheimer’s Disease transgenic mice as tissue-resident memory T cells. J Immunol. 2022;209:1272–1285. doi: 10.4049/jimmunol.2100737. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

