Surgical suction filter-derived bone graft displays osteogenic miRNA and mRNA patterns
- PMID: 37646799
- PMCID: PMC10923964
- DOI: 10.1007/s00068-023-02350-5
Surgical suction filter-derived bone graft displays osteogenic miRNA and mRNA patterns
Abstract
Purpose: Recently, a surgical suction filter device was introduced which aims at generating a suction filter-derived bone grafting substitute (SF-BGS). The osteogenic capacity of this grafting material, however, is unclear. MicroRNAs (miRNAs) and osteogenic mRNAs may influence these processes. The aim of this study was therefore to investigate the quality of the SF-BGS by determining the expression of miRNAs and osteogenic mRNAs.
Methods: Samples were collected during non-union surgery. Upon exposure of the intramedullary canal, the surgical vacuum system was fitted with the suction filter device containing collagen complex and synthetic β-TCP: (Ca3(PO4)2, granule size 5-8 mm, total volume 10 mL (Cerasorb Foam®, Curasan AG, Kleinostheim, Germany). As a control, venous blood was used as in current clinical practice. Samples were snap-frozen and mechanically disrupted. MiRNAs and mRNAs were isolated, transcribed, and pooled for qPCR analysis. Lastly, mRNA targets were determined through in silico target analyses.
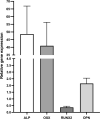
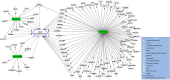
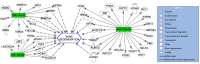
Results: The study population consisted of seven patients with a posttraumatic long bone non-union (4♀; mean age 54 ± 16 years). From the array data, distinct differences in miRNA expression were found between the SF-BGS and control samples. Osteogenic marker genes were overall upregulated in the SF-BGS. Qiagen IPA software identified 1168 mRNA targets for 43 of the overall deregulated miRNAs.
Conclusion: This study revealed distinctly deregulated and exclusively expressed osteogenic miRNAs in SF-BGS, as well as overall enhanced osteogenic marker gene expression, as compared to the venous blood control group. These expression profiles were not seen in control samples, indicating that the derived material displays an osteogenic profile. It may therefore be a promising tool to generate a BGS or graft extender when needed.
Keywords: Bone grafting substitute; Bone regeneration; Graft extender; Non-union; Stem cells; microRNAs.
© 2023. The Author(s).
Conflict of interest statement
All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures






Similar articles
-
Comprehensive analysis of lncRNA-miRNA-mRNA networks during osteogenic differentiation of bone marrow mesenchymal stem cells.BMC Genomics. 2022 Jun 7;23(1):425. doi: 10.1186/s12864-022-08646-x. BMC Genomics. 2022. PMID: 35672672 Free PMC article.
-
Osteogenic protein-1 for long bone nonunion: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(6):1-57. Epub 2005 Apr 1. Ont Health Technol Assess Ser. 2005. PMID: 23074475 Free PMC article.
-
Effects of a bone graft substitute consisting of porous gradient HA/ZrO2 and gelatin/chitosan slow-release hydrogel containing BMP-2 and BMSCs on lumbar vertebral defect repair in rhesus monkey.J Tissue Eng Regen Med. 2018 Mar;12(3):e1813-e1825. doi: 10.1002/term.2601. Epub 2017 Dec 26. J Tissue Eng Regen Med. 2018. PMID: 29055138
-
MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31.Gene. 2013 Sep 15;527(1):321-31. doi: 10.1016/j.gene.2013.06.021. Epub 2013 Jul 1. Gene. 2013. PMID: 23827457
-
The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation.Cells. 2019 Nov 19;8(11):1465. doi: 10.3390/cells8111465. Cells. 2019. PMID: 31752361 Free PMC article. Review.
Cited by
-
Surgical Site-Released Tissue Is Potent to Generate Bone onto TCP and PCL-TCP Scaffolds In Vitro.Int J Mol Sci. 2023 Nov 1;24(21):15877. doi: 10.3390/ijms242115877. Int J Mol Sci. 2023. PMID: 37958857 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

