Fibroblast Growth Factor 11 Inhibits Hepatitis B Virus Gene Expression Through FXRα Suppression
- PMID: 37646922
- PMCID: PMC10477102
- DOI: 10.1007/s12275-023-00065-1
Fibroblast Growth Factor 11 Inhibits Hepatitis B Virus Gene Expression Through FXRα Suppression
Abstract
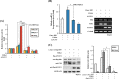
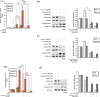
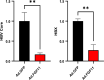
Fibroblast growth factor 11 (FGF11) is a member of the intracellular FGF family, which shows different signal transmission compared with other FGF superfamily members. The molecular function of FGF11 is not clearly understood. In this study, we identified the inhibitory effect of FGF11 on hepatitis B virus (HBV) gene expression through transcriptional suppression. FGF11 decreased the mRNA and protein expression of HBV genes in liver cells. While the nuclear receptor FXRα1 increased HBV promoter transactivation, FGF11 decreased the FXRα-mediated gene induction of the HBV promoter by the FXRα agonist. Reduced endogenous levels of FXRα by siRNA and the dominant negative mutant protein (aa 1-187 without ligand binding domain) of FXRα expression indicated that HBV gene suppression by FGF11 is dependent on FXRα inhibition. In addition, FGF11 interacts with FXRα protein and reduces FXRα protein stability. These results indicate that FGF11 inhibits HBV replicative expression through the liver cell-specific transcription factor, FXRα, and suppresses HBV promoter activity. Our findings may contribute to the establishment of better regimens for the treatment of chronic HBV infections by including FGF11 to alter the bile acid mediated FXR pathway.
Keywords: Antiviral effect; FGF11; FXR; HBx protein; Hepatitis B virus.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

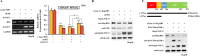


Similar articles
-
Farnesoid X receptor-α is a proviral host factor for hepatitis B virus that is inhibited by ligands in vitro and in vivo.FASEB J. 2019 Feb;33(2):2472-2483. doi: 10.1096/fj.201801181R. Epub 2018 Oct 11. FASEB J. 2019. PMID: 30307769
-
Transactivation of the hepatitis B virus core promoter by the nuclear receptor FXRalpha.J Virol. 2008 Nov;82(21):10832-40. doi: 10.1128/JVI.00883-08. Epub 2008 Sep 3. J Virol. 2008. PMID: 18768987 Free PMC article.
-
The metabolic sensors FXRα, PGC-1α, and SIRT1 cooperatively regulate hepatitis B virus transcription.FASEB J. 2014 Mar;28(3):1454-63. doi: 10.1096/fj.13-236372. Epub 2013 Dec 2. FASEB J. 2014. PMID: 24297698
-
Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha.J Nat Med. 2016 Jul;70(3):584-91. doi: 10.1007/s11418-016-0980-6. Epub 2016 Mar 11. J Nat Med. 2016. PMID: 26968537
-
Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition.Biochem Pharmacol. 2013 Oct 1;86(7):926-39. doi: 10.1016/j.bcp.2013.07.022. Epub 2013 Aug 6. Biochem Pharmacol. 2013. PMID: 23928191
References
-
- Befani C, Mylonis I, Gkotinakou IM, Georgoulias P, Hu CJ, Simos G, Liakos P. Cobalt stimulates HIF-1-dependent but inhibits HIF-2-dependent gene expression in liver cancer cell. The International Journal of Biochemistry and Cell Biology. 2013;45:2359–2368. doi: 10.1016/j.biocel.2013.07.025. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

