Modification of the GRACE Risk Score for Risk Prediction in Patients With Acute Coronary Syndromes
- PMID: 37647046
- PMCID: PMC10469286
- DOI: 10.1001/jamacardio.2023.2741
Modification of the GRACE Risk Score for Risk Prediction in Patients With Acute Coronary Syndromes
Erratum in
-
Corrected to Open Access Status.JAMA Cardiol. 2024 Jan 1;9(1):95. doi: 10.1001/jamacardio.2023.4300. JAMA Cardiol. 2024. PMID: 37938826 Free PMC article. No abstract available.
Abstract
Importance: The Global Registry of Acute Coronary Events (GRACE) risk score, a guideline-recommended risk stratification tool for patients presenting with acute coronary syndromes (ACS), does not consider the extent of myocardial injury.
Objective: To assess the incremental predictive value of a modified GRACE score incorporating high-sensitivity cardiac troponin (hs-cTn) T at presentation, a surrogate of the extent of myocardial injury.
Design, setting, and participants: This retrospectively designed longitudinal cohort study examined 3 independent cohorts of 9803 patients with ACS enrolled from September 2009 to December 2017; 2 ACS derivation cohorts (Heidelberg ACS cohort and Newcastle STEMI cohort) and an ACS validation cohort (SPUM-ACS study). The Heidelberg ACS cohort included 2535 and the SPUM-ACS study 4288 consecutive patients presenting with a working diagnosis of ACS. The Newcastle STEMI cohort included 2980 consecutive patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Data were analyzed from March to June 2023.
Exposures: In-hospital, 30-day, and 1-year mortality risk estimates derived from an updated risk score that incorporates continuous hs-cTn T at presentation (modified GRACE).
Main outcomes and measures: The predictive value of continuous hs-cTn T and modified GRACE risk score compared with the original GRACE risk score. Study end points were all-cause mortality during hospitalization and at 30 days and 1 year after the index event.
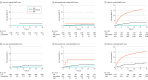
Results: Of 9450 included patients, 7313 (77.4%) were male, and the mean (SD) age at presentation was 64.2 (12.6) years. Using continuous rather than binary hs-cTn T conferred improved discrimination and reclassification compared with the original GRACE score (in-hospital mortality: area under the receiver operating characteristic curve [AUC], 0.835 vs 0.741; continuous net reclassification improvement [NRI], 0.208; 30-day mortality: AUC, 0.828 vs 0.740; NRI, 0.312; 1-year mortality: AUC, 0.785 vs 0.778; NRI, 0.078) in the derivation cohort. These findings were confirmed in the validation cohort. In the pooled population of 9450 patients, modified GRACE risk score showed superior performance compared with the original GRACE risk score in terms of reclassification and discrimination for in-hospital mortality end point (AUC, 0.878 vs 0.780; NRI, 0.097), 30-day mortality end point (AUC, 0.858 vs 0.771; NRI, 0.08), and 1-year mortality end point (AUC, 0.813 vs 0.797; NRI, 0.056).
Conclusions and relevance: In this study, using continuous rather than binary hs-cTn T at presentation, a proxy of the extent of myocardial injury, in the GRACE risk score improved the mortality risk prediction in patients with ACS.
Conflict of interest statement
Figures

References
-
- Szummer K, Wallentin L, Lindhagen L, et al. . Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995-2014. Eur Heart J. 2017;38(41):3056-3065. doi:10.1093/eurheartj/ehx515 - DOI - PMC - PubMed
-
- Szummer K, Wallentin L, Lindhagen L, et al. . Relations between implementation of new treatments and improved outcomes in patients with non-ST-elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J. 2018;39(42):3766-3776. doi:10.1093/eurheartj/ehy554 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

