Atherosclerotic Coronary Plaque Regression and Risk of Adverse Cardiovascular Events: A Systematic Review and Updated Meta-Regression Analysis
- PMID: 37647074
- PMCID: PMC10469293
- DOI: 10.1001/jamacardio.2023.2731
Atherosclerotic Coronary Plaque Regression and Risk of Adverse Cardiovascular Events: A Systematic Review and Updated Meta-Regression Analysis
Abstract
Importance: The association between changes in atherosclerotic plaque induced by lipid-lowering therapies (LLTs) and reduction in major adverse cardiovascular events (MACEs) remains controversial.
Objective: To evaluate the association between coronary plaque regression assessed by intravascular ultrasound (IVUS) and MACEs.
Data sources: A comprehensive, systematic search of publications in PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science was performed.
Study selection: Clinical prospective studies of LLTs reporting change in percent atheroma volume (PAV) assessed by IVUS and describing MACE components were selected.
Data extraction and synthesis: Reporting was performed in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The association between mean change in PAV and MACEs was analyzed by meta-regression using mixed-effects, 2-level binomial logistic regression models, unadjusted and adjusted for clinical covariates, including mean age, baseline PAV, baseline low-density lipoprotein cholesterol level, and study duration.
Main outcome and measures: Mean PAV change and MACE in intervention and comparator arms were assessed in an updated systematic review and meta-regression analysis of IVUS trials of LLTs that also reported MACEs.
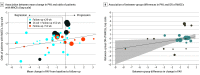
Results: This meta-analysis included 23 studies published between July 2001 and July 2022, including 7407 patients and trial durations ranging from 11 to 104 weeks. Mean (SD) patient age ranged from 55.8 (9.8) to 70.2 (7.6) years, and the number of male patients from 245 of 507 (48.3%) to 24 of 26 (92.3%). Change in PAV across 46 study arms ranged from -5.6% to 3.1%. The number of MACEs ranged from 0 to 72 per study arm (17 groups [37%] reported no events, 9 [20%] reported 1-2 events, and 20 [43%] reported ≥3 events). In unadjusted analysis, a 1% decrease in mean PAV was associated with 17% reduced odds of MACEs (unadjusted OR, 0.83; 95% CI, 0.71-0.98; P = .03), and with a 14% reduction in MACEs in adjusted analysis (adjusted OR, 0.86; 95% CI, 0.75-1.00; P = .050). Further adjustment for cardiovascular risk factors showed a 19% reduced risk (adjusted OR, 0.81; 95% CI, 0.68-0.96; P = .01) per 1% decrease in PAV. A 1% reduction of PAV change between intervention and comparator arms within studies was also associated with a significant 25% reduction in MACEs (OR, 0.75; 95% CI, 0.56-1.00; P = .046).
Conclusions and relevance: In this meta-analysis, regression of atherosclerotic plaque by 1% was associated with a 25% reduction in the odds of MACEs. These findings suggest that change in PAV could be a surrogate marker for MACEs, but given the heterogeneity in the outcomes, additional data are needed.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

