Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child
- PMID: 37647086
- PMCID: PMC10463554
- DOI: 10.1002/14651858.CD010224.pub3
Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child
Abstract
Background: Prenatal exposure to certain anti-seizure medications (ASMs) is associated with an increased risk of major congenital malformations (MCM). The majority of women with epilepsy continue taking ASMs throughout pregnancy and, therefore, information on the potential risks associated with ASM treatment is required.
Objectives: To assess the effects of prenatal exposure to ASMs on the prevalence of MCM in the child.
Search methods: For the latest update of this review, we searched the following databases on 17 February 2022: Cochrane Register of Studies (CRS Web), MEDLINE (Ovid, 1946 to February 16, 2022), SCOPUS (1823 onwards), and ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP). No language restrictions were imposed.
Selection criteria: We included prospective cohort controlled studies, cohort studies set within pregnancy registries, randomised controlled trials and epidemiological studies using routine health record data. Participants were women with epilepsy taking ASMs; the two control groups were women without epilepsy and untreated women with epilepsy.
Data collection and analysis: Five authors independently selected studies for inclusion. Eight authors completed data extraction and/or risk of bias assessments. The primary outcome was the presence of an MCM. Secondary outcomes included specific types of MCM. Where meta-analysis was not possible, we reviewed included studies narratively.
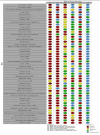
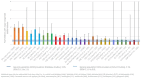
Main results: From 12,296 abstracts, we reviewed 283 full-text publications which identified 49 studies with 128 publications between them. Data from ASM-exposed pregnancies were more numerous for prospective cohort studies (n = 17,963), than data currently available for epidemiological health record studies (n = 7913). The MCM risk for children of women without epilepsy was 2.1% (95% CI 1.5 to 3.0) in cohort studies and 3.3% (95% CI 1.5 to 7.1) in health record studies. The known risk associated with sodium valproate exposure was clear across comparisons with a pooled prevalence of 9.8% (95% CI 8.1 to 11.9) from cohort data and 9.7% (95% CI 7.1 to 13.4) from routine health record studies. This was elevated across almost all comparisons to other monotherapy ASMs, with the absolute risk differences ranging from 5% to 9%. Multiple studies found that the MCM risk is dose-dependent. Children exposed to carbamazepine had an increased MCM prevalence in both cohort studies (4.7%, 95% CI 3.7 to 5.9) and routine health record studies (4.0%, 95% CI 2.9 to 5.4) which was significantly higher than that for the children born to women without epilepsy for both cohort (RR 2.30, 95% CI 1.47 to 3.59) and routine health record studies (RR 1.14, 95% CI 0.80 to 1.64); with similar significant results in comparison to the children of women with untreated epilepsy for both cohort studies (RR 1.44, 95% CI 1.05 to 1.96) and routine health record studies (RR 1.42, 95% CI 1.10 to 1.83). For phenobarbital exposure, the prevalence was 6.3% (95% CI 4.8 to 8.3) and 8.8% (95% CI 0.0 to 9277.0) from cohort and routine health record data, respectively. This increased risk was significant in comparison to the children of women without epilepsy (RR 3.22, 95% CI 1.84 to 5.65) and those born to women with untreated epilepsy (RR 1.64, 95% CI 0.94 to 2.83) in cohort studies; data from routine health record studies was limited. For phenytoin exposure, the prevalence of MCM was elevated for cohort study data (5.4%, 95% CI 3.6 to 8.1) and routine health record data (6.8%, 95% CI 0.1 to 701.2). The prevalence of MCM was higher for phenytoin-exposed children in comparison to children of women without epilepsy (RR 3.81, 95% CI 1.91 to 7.57) and the children of women with untreated epilepsy (RR 2.01. 95% CI 1.29 to 3.12); there were no data from routine health record studies. Pooled data from cohort studies indicated a significantly increased MCM risk for children exposed to lamotrigine in comparison to children born to women without epilepsy (RR 1.99, 95% CI 1.16 to 3.39); with a risk difference (RD) indicating a 1% increased risk of MCM (RD 0.01. 95% CI 0.00 to 0.03). This was not replicated in the comparison to the children of women with untreated epilepsy (RR 1.04, 95% CI 0.66 to 1.63), which contained the largest group of lamotrigine-exposed children (> 2700). Further, a non-significant difference was also found both in comparison to the children of women without epilepsy (RR 1.19, 95% CI 0.86 to 1.64) and children born to women with untreated epilepsy (RR 1.00, 95% CI 0.79 to 1.28) from routine data studies. For levetiracetam exposure, pooled data provided similar risk ratios to women without epilepsy in cohort (RR 2.20, 95% CI 0.98 to 4.93) and routine health record studies (RR 0.67, 95% CI 0.17 to 2.66). This was supported by the pooled results from both cohort (RR 0.71, 95% CI 0.39 to 1.28) and routine health record studies (RR 0.82, 95% CI 0.39 to 1.71) when comparisons were made to the offspring of women with untreated epilepsy. For topiramate, the prevalence of MCM was 3.9% (95% CI 2.3 to 6.5) from cohort study data and 4.1% (0.0 to 27,050.1) from routine health record studies. Risk ratios were significantly higher for children exposed to topiramate in comparison to the children of women without epilepsy in cohort studies (RR 4.07, 95% CI 1.64 to 10.14) but not in a smaller comparison to the children of women with untreated epilepsy (RR 1.37, 95% CI 0.57 to 3.27); few data are currently available from routine health record studies. Exposure in utero to topiramate was also associated with significantly higher RRs in comparison to other ASMs for oro-facial clefts. Data for all other ASMs were extremely limited. Given the observational designs, all studies were at high risk of certain biases, but the biases observed across primary data collection studies and secondary use of routine health records were different and were, in part, complementary. Biases were balanced across the ASMs investigated, and it is unlikely that the differential results observed across the ASMs are solely explained by these biases.
Authors' conclusions: Exposure in the womb to certain ASMs was associated with an increased risk of certain MCMs which, for many, is dose-dependent.
Antecedentes: La exposición prenatal a determinados fármacos anticonvulsivos (FAC) se asocia con un mayor riesgo de malformaciones congénitas graves (MCG). La mayoría de las mujeres con epilepsia continúan tomando FAC durante todo el embarazo y, por lo tanto, se requiere información sobre los riesgos potenciales asociados con el tratamiento con FAC.
Objetivos: Evaluar los efectos de la exposición prenatal a los FAC sobre la prevalencia de MCG en el niño. MÉTODOS DE BÚSQUEDA: Para la última actualización de esta revisión se hicieron búsquedas el 17 de febrero de 2022 en las siguientes bases de datos: Registro Cochrane de Estudios (Cochrane Register of Studies [CRS Web]), MEDLINE (Ovid, 1946 hasta el 16 de febrero de 2022), SCOPUS (1823 en adelante) y ClinicalTrials.gov , Plataforma de registros internacionales de ensayos clínicos (ICTRP). No se impusieron restricciones de idioma. CRITERIOS DE SELECCIÓN: Se incluyeron estudios prospectivos controlados de cohortes, estudios de cohortes establecidos dentro de registros de embarazos, ensayos controlados aleatorizados y estudios epidemiológicos que utilizaron datos rutinarios de los historiales médicos. Las participantes fueron mujeres con epilepsia que tomaban FAC; los dos grupos de control fueron mujeres sin epilepsia y mujeres con epilepsia que no recibían tratamiento. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Cinco autores seleccionaron de forma independiente los estudios para inclusión. Ocho autores completaron la extracción de los datos y las evaluaciones del riesgo de sesgo. El desenlace principal fue la presencia de una MCG. Los desenlaces secundarios incluyeron tipos específicos de MCG. Cuando no fue posible realizar un metanálisis, los estudios incluidos se examinaron de forma narrativa.
Resultados principales: De 12 296 resúmenes, se revisaron 283 publicaciones a texto completo que identificaron 49 estudios con 128 publicaciones entre ellos. Los datos de los embarazos expuestos a FAC fueron más numerosos en el caso de los estudios prospectivos de cohortes (n = 17 963), que los datos actualmente disponibles de estudios de registros sanitarios epidemiológicos (n = 7913). El riesgo de MCG en los hijos de mujeres sin epilepsia fue del 2,1% (IC del 95%: 1,5 a 3,0) en los estudios de cohortes y del 3,3% (IC del 95%: 1,5 a 7,1) en los estudios de registros sanitarios. El riesgo conocido asociado con la exposición al valproato de sodio fue evidente en todas las comparaciones, con una prevalencia agrupada del 9,8% (IC del 95%: 8,1 a 11,9) a partir de los datos de los estudios de cohortes y del 9,7% (IC del 95%: 7,1 a 13,4) a partir de los estudios con datos rutinarios de los historiales médicos. Este fue elevado en casi todas las comparaciones con otros FAC como monoterapia, con diferencias absolutas de riesgo que variaron entre el 5% y el 9%. Múltiples estudios han constatado que el riesgo de MCG depende de la dosis. Los niños expuestos a la carbamazepina tuvieron una mayor prevalencia de MCG tanto en los estudios de cohortes (4,7%; IC del 95%: 3,7 a 5,9) como en los estudios con datos rutinarios de los historiales médicos (4,0%; IC del 95%: 2,9 a 5,4), que fue significativamente superior a la de los niños nacidos de mujeres sin epilepsia tanto en los estudios de cohortes (RR 2,30; IC del 95%: 1,47 a 3,59) como en los estudios de historias clínicas habituales (RR 1,14; IC del 95%: 0,80 a 1,64), con resultados significativos similares en comparación con los hijos de mujeres con epilepsia que no reciben tratamiento tanto en los estudios de cohortes (RR 1,44; IC del 95%: 1,05 a 1,96) como en los estudios con datos rutinarios de los historiales médicos (RR 1,42; IC del 95%: 1,10 a 1,83). Para la exposición al fenobarbital, la prevalencia fue del 6,3% (IC del 95%: 4,8 a 8,3) y del 8,8% IC del 95%: 0,0 a 9277,0) a partir de los datos de estudios de cohortes y los datos de estudios con datos rutinarios de los historiales médicos, respectivamente. Este aumento del riesgo fue significativo en comparación con los hijos de mujeres sin epilepsia (RR 3,22; IC del 95%: 1,84 a 5,65) y los nacidos de mujeres con epilepsia que no reciben tratamiento (RR 1,64; IC del 95%: 0,94 a 2,83) en estudios de cohortes; los datos procedentes de estudios con datos rutinarios de los historiales médicos fueron limitados. En cuanto a la exposición a la fenitoína, la prevalencia de MCG fue elevada en los datos de los estudios de cohortes (5,4%; IC del 95%: 3,6 a 8,1) y en los datos rutinarios de los historiales médicos (6,8%; IC del 95%: 0,1 a 701,2). La prevalencia de MCG fue mayor en los niños expuestos a la fenitoína en comparación con los hijos de mujeres sin epilepsia (RR 3,81; IC del 95%: 1,91 a 7,57) y los hijos de mujeres con epilepsia que no reciben tratamiento (RR 2,01; IC del 95%: 1,29 a 3,12); no hubo datos procedentes de estudios con datos rutinarios de los historiales médicos. Los datos agrupados de los estudios de cohortes indicaron un riesgo significativamente mayor de MCG en los niños expuestos a lamotrigina en comparación con los niños nacidos de mujeres sin epilepsia (RR 1,99; IC del 95%: 1,16 a 3,39); con una diferencia de riesgos (DR) que indica un riesgo 1% mayor de MCG (DR 0,01. IC del 95%: 0,00 a 0,03). Esto no se repitió en la comparación con los hijos de las mujeres con epilepsia que no reciben tratamiento (RR 1,04; IC del 95%: 0,66 a 1,63), que contenía el mayor grupo de niños expuestos a la lamotrigina (> 2700). Además, también se encontró una diferencia no significativa tanto en comparación con los hijos de mujeres sin epilepsia (RR 1,19; IC del 95%: 0,86 a 1,64) como con los hijos de mujeres con epilepsia que no reciben tratamiento (RR 1,00; IC del 95%: 0,79 a 1,28) a partir de los estudios con datos rutinarios. Para la exposición al levetiracetam, los datos agrupados proporcionaron razones de riesgos similares a las de las mujeres sin epilepsia en los estudios de cohortes (RR 2,20; IC del 95%: 0,98 a 4,93) y en los estudios con datos rutinarios de los historiales médicos (RR 0,67; IC del 95%: 0,17 a 2,66). Los resultados agrupados de los estudios de cohortes (RR: 0,71; IC del 95%: 0,39 a 1,28) y de los estudios con datos rutinarios de los historiales médicos (RR: 0,82; IC del 95%: 0,39 a 1,71) respaldan esta afirmación cuando se comparan con los hijos de las mujeres con epilepsia que no reciben tratamiento. En el caso del topiramato, la prevalencia de MCG fue del 3,9% (IC del 95%: 2,3 a 6,5) a partir de los datos de los estudios de cohortes y del 4,1% (0,0 a 27.050,1) a partir de los estudios con datos rutinarios de los historiales médicos. Las razones de riesgos fueron significativamente más altas para los niños expuestos al topiramato en comparación con los hijos de mujeres sin epilepsia en estudios de cohortes (RR 4,07; IC del 95%: 1,64 a 10,14), pero no en una comparación más pequeña con los hijos de mujeres con epilepsia que no reciben tratamiento (RR 1,37; IC del 95%: 0,57 a 3,27); actualmente se dispone de pocos datos a partir de estudios con datos rutinarios de los historiales médicos. La exposición en el útero al topiramato también se asoció con RR significativamente mayores en comparación con otros FAC para las hendiduras orofaciales. Los datos de todos las demás FAC fueron extremadamente limitados. Debido a los diseños observacionales, todos los estudios presentaron un alto riesgo de ciertos sesgos, pero los sesgos observados en los estudios de obtención de datos primarios y el uso secundario de historiales médicos rutinarios fueron diferentes y, en parte, complementarios. Los sesgos estaban equilibrados entre los FAC investigados, y es poco probable que los resultados diferenciales observados entre los FAC se expliquen únicamente por estos sesgos.
Conclusiones de los autores: La exposición en el útero a ciertos FAC se asoció con un mayor riesgo de ciertos MCG que, para muchos, depende de la dosis.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
RB's institution has received consultancy fees from UCB Pharma on one occasion due to work undertaken by RB.
NA has been sponsored to attend educational meetings and conferences in epilepsy over the last five years by UCB Pharma, GSK and Boehringer Ingelheim, and has participated in regional advisory Board meetings for Eisai on their product eslicarbazepine and zonisamide.
AM leads the National Audit of Seizure Management in Hospitals (NASH), which is funded via a grant from UCB Pharma paid to the University of Liverpool. He has also given lectures at educational events sponsored by Sanofi and GSK, with honoraria paid to University of Liverpool. Professor Tony Marson is Theme Leader for Managing Complex Needs at NIHR CLAHRC NWC and an NIHR Senior Investigator.
No other conflicts of interest were declared.
Figures










































































































































































































































































































































































Update of
-
Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child.Cochrane Database Syst Rev. 2016 Nov 7;11(11):CD010224. doi: 10.1002/14651858.CD010224.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2023 Aug 29;8:CD010224. doi: 10.1002/14651858.CD010224.pub3. PMID: 27819746 Free PMC article. Updated.
References
References to studies included in this review
Al Bunyan 1999 {published data only}
-
- Al Bunyan M, Abo-Talib Z. Outcome of pregnancies in epileptic women: a study in Saudi Arabia. Seizure 1999;8(1):26-9. [PMID: ] - PubMed
AlSheikh 2020 {published data only}
Australian Epilepsy and Pregnancy Register {published and unpublished data}
-
- Jazayeri D, Graham J, Hitchcock A, O'Brien TJ, Vajda FJ. Outcomes of pregnancies in women taking antiepileptic drugs for non-epilepsy indications. Seizure 2018;56:111-4. [PMID: ] - PubMed
-
- Vajda F, Lander C, O'Brien T, Hitchcock A, Graham J, Solinas C, et al. Australian pregnancy registry of women taking antiepileptic drugs. Epilepsia 2004;45(11):1466. [PMID: ] - PubMed
-
- Vajda F, O'Brien T, Graham J, Lander C, Eadie M. Dose dependence of fetal malformations associated with valproate. Neurology 2013;81(11):999-1003. [PMID: ] - PubMed
-
- Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurologica Scandinavica 2005;112(3):137-43. [PMID: ] - PubMed
-
- Vajda FJ, Graham J, Lander CM, O'Brien TJ, Eadie M. Teratogenicity of the newer antiepileptic drugs - the Australian experience. Journal of Clinical Neuroscience 2012;19(1):57-9. [PMID: ] - PubMed
Bag 1989 {published data only}
-
- Bag S, Behari M, Ahuja GK, Karmarkar MG. Pregnancy and epilepsy. Journal of Neurology 1989;236(5):311-3. [PMID: ] - PubMed
Barqawi 2005 {published data only}
-
- Barqawi R. Evaluation of antiepileptic drugs in pregnancy in a Jordanian army hospital. Eastern Mediterranean Health Journal 2005;11(4):601-5. [PMID: ] - PubMed
Cassina 2013 {published data only}
-
- Cassina M, Dilaghi A, Di Gianantonio E, Cesari E, De Santis M, Mannaioni G, et al. Pregnancy outcome in women exposed to antiepileptic drugs: teratogenic role of maternal epilepsy and its pharmacologic treatment. Reproductive Toxicology 2013;39:50-7. [PMID: ] - PubMed
D'Souza 1991 {published data only}
Delmiš 1991 {published data only}
-
- Đelmiš J, Dražančić A, Tkalčević T, Ivanišević M. Epilepsy in pregnancy [Epilepsija i trudnoća]. Jugoslavenska Ginekologija i Perinatologija 1991;31(1-2):23-6. [PMID: ] - PubMed
Denmark Health Record Registers {published and unpublished data}
-
- Christensen J, Trabjerg BB, Sun Y, Gilhus NE, Bjork M-H, Tomson T, et al. Prenatal exposure to valproate and risk of congenital malformations - could we have known earlier? A population-based cohort study. Epilepsia 2021;62(12):2981-93. [PMID: ] - PubMed
-
- Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305(19):1996-2002. [PMID: ] - PubMed
Eroglu 2008 {published data only}
-
- Eroğlu E, Gökçil Z, Bek S, Ulaş UH, Odabaşi Z. Pregnancy and teratogenicity of antiepileptic drugs. Acta Neurologica Belgica 2008;108(2):53-7. [PMID: ] - PubMed
EURAP 2018 {published data only}
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurology 2018;17(6):530-8. [PMID: ] - PubMed
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Declining malformation rates with changed antiepileptic drug prescribing: an observational study. Neurology 2019;93(9):e831-40. [PMID: ] - PubMed
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology 2015;85(10):866-72. [PMID: ] - PubMed
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurology 2011;10(7):609-17. [PMID: ] - PubMed
Fairgrieve 2000 {published data only}
Finland Health Record Registers {published data only}
-
- Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology 2005;64(11):1874-8. [PMID: ] - PubMed
-
- Artama M, Ritvanen A, Gissler M, Isojärvi J, Auvinen A. Congenital structural anomalies in offspring of women with epilepsy - a population-based cohort study in Finland. International Journal of Epidemiology 2006;35(2):280-7. [PMID: ] - PubMed
Fröscher 1991 {published data only}
-
- Fröscher W, Herrmann R, Niesen M, Bülau P, Penin H, Hildenbrand G. The course of pregnancy and teratogenicity of antiepileptic agents in 66 patients with epilepsy [Untersuchungen zum schwangerschaftsverlauf und zur teratogenität der antiepileptika bei 66 epilepsie-patientinnen]. Schweizer Archiv fur Neurologie und Psychiatrie 1991;142(5):389-407. [PMID: ] - PubMed
Garza‐Morales 1996 {published data only}
-
- Garza-Morales S, Ibarra-Puig JM, Poblano-Luna A, Gilda Mayén-Molina D, Córdova-López S. Epilepsy and pregnancy: prospective study of 100 cases [Epilepsia y embarazo. Estudio prospectivo 100 casos]. Ginecología y Obstetricia de México 1996;64:449-54. [PMID: ] - PubMed
Hosny 2021 {published data only}
-
- Hosny H, Elkattan M, Zaki MA, Ramzy GM, Magdy R, Abo Al-Azayem S. Risk factors of fetal deaths and major birth defects in newborns of women with epilepsy: an Egyptian prospective study. Epilepsy & Behavior 2021;123:108251. [PMID: ] - PubMed
Israeli Teratogen Service {published data only}
-
- Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Is carbamazepine teratogenic? A prospective controlled study of 210 pregnancies. Neurology 2001;57(2):321-4. [PMID: ] - PubMed
-
- Diav-Citrin O, Shechtman S, Bar-Oz B, Cantrell D, Arnon J, Ornoy A. Pregnancy outcome after in utero exposure to valproate: evidence of dose relationship in teratogenic effect. CNS Drugs 2008;22(4):325-34. [PMID: ] - PubMed
-
- Diav-Citrin O, Shechtman S, Zvi N, Finkel-Pekarsky V, Ornoy A. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Research 2017;109(15):1196-1203. [PMID: ] - PubMed
-
- Ornoy A, Zvi N, Arnon J, Wajnberg R, Shechtman S, Diav-Citrin O. The outcome of pregnancy following topiramate treatment: a study on 52 pregnancies. Reproductive Toxicology 2008;25(3):388-9. [PMID: ] - PubMed
Italian Lombardy Region Health Register {published data only}
-
- Putignano D, Clavenna A, Campi R, Canevini MP, Vignoli A, Battino D, et al. Perinatal outcome and healthcare resource utilization in the first year of life after antiepileptic exposure during pregnancy. Epilepsy & Behavior 2019;92:14-7. [PMID: ] - PubMed
Jimenez 2020 {published data only}
-
- Jiménez M, Grau-López L, Ciurans J, García-Esperón C, Fumanal A, Barambio S, Chíes E, Codina M, Becerra JL. Epilepsy and pregnancy. Factors associated with epileptic seizures during pregnancy. Neurologia (Engl Ed) 2020 [Epub ahead of print];38(2):106-113. [DOI: 10.1016/j.nrl.2020.04.024] [PMID: ] - DOI - PubMed
Kaaja 2003 {published data only}
-
- Kaaja E, Kaaja R, Hiilesmaa V. Major malformations in offspring of women with epilepsy. Neurology 2003;60(4):575-9. [PMID: ] - PubMed
Kaneko 1999 {published data only}
-
- Fukushima Y, Nakamura Y, Ogawa Y, Saito Y, Kan R, Kumashiro H, et al. Teratogenicity of antiepileptic drugs: is the prevention possible? Japanese Journal of Psychiatry and Neurology 1991;45(2):478-81. [PMID: ] - PubMed
-
- Kaneko S, Battino D, Andermann E, Wada K, Kan R, Takeda A, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Research 1999;33(2-3):141-58. [PMID: ] - PubMed
-
- Kaneko S, Otani K, Fukushima Y, Ogawa Y, Nomura Y, Ono T, et al. Teratogenicity of antiepileptic drugs: analysis of possible risk factors. Epilepsia 1988;29(4):459-67. [PMID: ] - PubMed
-
- Kaneko S, Otani K, Kondo T, Fukushima Y, Kan R, Takeda A, et al. Teratogenicity of antiepileptic drugs and drug specific malformations. Japanese Journal of Psychiatry and Neurology 1993;47(2):306-8. [PMID: ] - PubMed
-
- Kaneko S, Otani K, Kondo T, Fukushima Y, Nakamura Y, Ogawa Y, et al. Malformation in infants of mothers with epilepsy receiving antiepileptic drugs. Neurology 1992;42(4 Suppl 5):68-74. [PMID: ] - PubMed
Kaur 2020 {published data only}
Kelly 1984 {published data only}
-
- Kelly TE, Edwards P, Rein M, Miller JQ, Dreifuss FE. Teratogenicity of anticonvulsant drugs II: a prospective study. American Journal of Medical Genetics 1984;19(3):435-43. [PMID: ] - PubMed
Kerala Epilepsy and Pregnancy Registry {published data only}
-
- Begum S, Sarma SP, Thomas SV. Malformation in index pregnancy in women with epilepsy is not followed by recurrence in subsequent pregnancy. Epilepsia 2013;54(12):e163-7. [PMID: ] - PubMed
-
- Keni RR, Jose M, Reshma AS, Baishya J, Sankara Sarma P, Thomas SV. Anti-epileptic drug and folic acid usage during pregnancy, seizure and malformation outcomes: changes over two decades in the Kerala Registry of Epilepsy and Pregnancy. Epilepsy Research 2020;159:106250. [PMID: ] - PubMed
-
- Keni RR, Jose M, Sarma PS, Thomas SV, Kerala Registry of Epilepsy and Pregnancy Study Group. Teratogenicity of antiepileptic dual therapy: dose-dependent, drug-specific, or both? Neurology 2018;90(9):e790-6. [PMID: ] - PubMed
-
- Seshachala BB, Jose M, Lathikakumari AM, Murali S, Kumar AS, Thomas SV. Valproate usage in pregnancy: an audit from the Kerala Registry of Epilepsy and Pregnancy. Epilepsia 2021;62(5):1141-7. [PMID: ] - PubMed
-
- Thomas SV, Ajaykumar B, Sindhu K, Francis E, Namboodiri N, Sivasankaran S, et al. Cardiac malformations are increased in infants of mothers with epilepsy. Pediatric Cardiology 2008;29(3):604-8. [PMID: ] - PubMed
Koch 1992 {published data only}
-
- Jäger-Roman E, Deichl A, Jakob S, Hartmann AM, Koch S, Rating D, et al. Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. Journal of Pediatrics 1986;108(6):997-1004. [PMID: ] - PubMed
-
- Koch S, Gopfert-Geyer I, Jager-Roman E, Jakob S, Huth H, Hartmann A, et al. Anti-epileptic agents during pregnancy: a prospective study on the course of pregnancy, malformations and child development [Antiepileptika wahrend der schwangerschaft. Eine prospektive studie uber schwangerschaftsverlauf, fehlbildungen und kindliche entwicklung]. Deutsche Medizinische Wochenschrift 1983;108(7):250-7. [PMID: ] - PubMed
-
- Koch S, Lösche G, Jager-Romän E, Jakob S, Rating D, Deichl A, et al. Major and minor birth malformations and antiepileptic drugs. Neurology 1992;42(4 Suppl 5):83-8. [PMID: ] - PubMed
-
- Kuhnz W, Jäger-Roman E, Rating D, Deichl A, Kunze J, Helge H, et al. Carbamazepine and carbamazepine-10, -11 epoxide during pregnancy and postnatal period in epileptic mother and their nursed infants: pharmacokinetics and clinical effects. Pediatric Pharmacology 1983;3(3-4):199-208. [PMID: ] - PubMed
Lindhout 1992 {published data only}
-
- Lindhout D, Höppener RJ, Meinardi H. Teratogenicity of antiepileptic drug combinations with special emphasis on epoxidation (of carbamazepine). Epilepsia 1984;25(1):77-83. [PMID: ] - PubMed
-
- Lindhout D, Meinardi H, Meijer JW, Nau H. Antiepileptic drugs and teratogenesis in two consecutive cohorts: changes in prescription policy paralleled by changes in pattern of malformations. Neurology 1992;42(4 Suppl 5):94-110. [PMID: ] - PubMed
Martinez Ferri 2018 {published data only}
-
- Martinez Ferri M, Pena Mayor P, Perez Lopez-Fraile I, Escartin Siquier A, Martin Moro M, Forcadas Berdusan M, et al. Comparative study of antiepileptic drug use during pregnancy over a period of 12 years in Spain. Efficacy of the newer antiepileptic drugs lamotrigine, levetiracetam, and oxcarbazepine. Neurologia 2018;33(2):78-84. [PMID: ] - PubMed
-
- Martinez Ferri M, Pena Mayor P, Perez Loppez-Fraile I, Castro Vilanova MD, Escartin Siquier A, Martin Moro M, et al. Malformations and fetal death in the Spanish antiepileptic drug and pregnancy registry: results at 6 years [Malformaciones y muerte fetal en el registro espanol de farmacos antiepilepticos y embarazo: resultados a los 6 anos]. Neurologia 2009;24(6):360-5. [PMID: ] - PubMed
Mawer 2010 {published data only}
Meador 2006 {published data only}
Meischenguiser 2004 {published data only}
-
- Meischenguiser R, D'Giano CH, Ferraro SM. Oxcarbazepine in pregnancy: clinical experience in Argentina. Epilepsy & Behaviour 2004;5(2):163-7. [PMID: ] - PubMed
Melikova 2020 {published data only}
-
- Melikova S, Bagirova H, Magalov S. The impact of maternal epilepsy on delivery and neonatal outcomes. Child's Nervous System 2020;36(4):775-82. [PMID: ] - PubMed
Milan Study 1999 {published data only}
-
- Battino D, Binelli S, Caccamo ML, Canevini MP, Canger R, Como ML, et al. Malformations in offspring of 305 epileptic women: a prospective study. Acta Neurologica Scandinavica 1992;85(3):204-7. [PMID: ] - PubMed
-
- Battino D, Kaneko S, Andermann E, Avanzini G, Canevini MP, Canger R, et al. Intrauterine growth in the offspring of epileptic women: a prospective multicentre study. Epilepsy Research 1999;36(1):53-60. [PMID: ] - PubMed
-
- Canger R, Battino D, Canevini MP, Fumarola C, Guidolin L, Vignoli A, et al. Malformations in offspring of women with epilepsy: a prospective study. Epilepsia 1999;40(9):1231-6. [PMID: ] - PubMed
Miskov 2016 {published data only}
-
- Miskov S, Gjergja Juraski R, Mikula I, Basic S, Bosnjak Pasic M, Kosec V, et al. The Croatian model of integrative prospective management of epilepsy and pregnancy. Acta Clinica Croatica 2016;55(4):535-48. [PMID: ] - PubMed
MONEAD 2020 {published data only}
Montreal Series {published data only}
-
- Dansky LV, Andermann E, Rosenblatt D, Sherwin AL, Andermann F. Anticonvulsants, folate levels, and pregnancy outcome: a prospective study. Annals of Neurology 1987;21(2):176-82. [PMID: ] - PubMed
-
- Oguni M, Dansky L, Andermann E, Sherwin A, Andermann F. Improved pregnancy outcome in epileptic women in the last decade: relationship to maternal anticonvulsant therapy. Brain & Development 1992;14(6):371-80. [PMID: ] - PubMed
Motherisk Registry {published data only}
-
- Gladstone DJ, Bologa M, Maguire C, Pastuszak A, Koren G. Course of pregnancy and fetal outcome following maternal exposure to carbamazepine and phenytoin: a prospective study. Reproductive Toxicology 1992;6(3):257-61. [PMID: ] - PubMed
-
- Nulman I, Scolnik D, Chitayat D, Farkas LD, Koren G. Findings in children exposed in utero to phenytoin and carbamazepine monotherapy: independent effects of epilepsy and medications. American Journal of Medical Genetics 1997;68(1):18-24. [PMID: ] - PubMed
North American Epilepsy and Pregnancy Register {published data only}
-
- Bokhari A, Coull BA, Holmes LB. Effect of prenatal exposure to anticonvulsant drugs on dermal ridge patterns of fingers. Teratology 2002;66(1):19-23. [PMID: ] - PubMed
-
- Bromfield EB, Dworetzky BA, Wyszynski DF, Smith CR, Baldwin EJ, Holmes LB. Valproate teratogenicity and epilepsy syndrome. Epilepsia 2008;49(12):2122-4. [PMID: ] - PubMed
-
- Hernandez-Diaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78(21):1692-9. [PMID: ] - PubMed
-
- Hernández-Díaz S, Mittendorf R, Smith CR, Hauser WA, Yerby M, Holmes LB, et al. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstetrics and Gynecology 2014;123(1):21-8. [PMID: ] - PubMed
-
- Holmes LB, Baldwin EJ, Smith CR, Habecker E, Glassman L, Wong SL, et al. Increased frequency of isolated cleft palate in infants exposed to lamotrigine during pregnancy. Neurology 2008;70(22 Pt 2):2152-8. [PMID: ] - PubMed
Norwegian Health Record Registers {published data only}
-
- Borthen I, Eide MG, Veiby G, Daltveit AK, Gilhus NE. Complications during pregnancy in women with epilepsy: population-based cohort study. British Journal of Obstetrics and Gynecology 2009;116(13):1736–42. [PMID: ] - PubMed
-
- Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. Journal of Neurology 2014;261(13):579-88. [PMID: ] - PubMed
-
- Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia 2009;50(9):2130-9. [PMID: ] - PubMed
Omtzigt 1992 {published data only}
-
- Omtzigt JG, Los FJ, Grobbee DE, Pijpers L, Jahoda MG, Brandenburg H, et al. The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology 1992;42(4 Suppl 5):119-25. [PMID: ] - PubMed
-
- Omtzigt JG, Los FJ, Hagenaars AM, Stewart PA, Sachs ES, Lindhout D. Prenatal diagnosis of spina bifida aperta after first-trimester valproate exposure. Prenatal Diagnosis 1992;12(11):893-7. [PMID: ] - PubMed
Pardi 1982 {published data only}
-
- Pardi G, Como ML, De Giambattista M, Oldrini A, Pifarotti G. Epilepsy and pregnancy: obstetrical aspects of a prospective multidisciplinary study [Epilessia e gravidanza: aspetti ostetrici di uno studio prospettico multidisciplinare]. Annali di Ostetricia, Ginecologia, Medicina Perinatale 1982;103(4):254-63. [PMID: ] - PubMed
Samren 1997 {published data only}
-
- Samrén EB, Van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia 1997;38(9):981-90. [PMID: ] - PubMed
Steegers‐Theunissen 1994 {published data only}
-
- Steegers-Theunissen RP, Renier WO, Borm GF, Thomas CM, Merkus HM, Op de Coul DA, et al. Factors influencing the risk of abnormal pregnancy outcome in epileptic women: a multi-centre prospective study. Epilepsy Research 1994;18(3):261-9. [PMID: ] - PubMed
Sweden Health Record Registers {published data only}
-
- Källén B. A register study of maternal epilepsy and delivery outcome with special reference to drug use. Acta Neurologica Scandinavica 1986;73(3):253-9. [PMID: ] - PubMed
-
- Wide K, Winbladh B, Källén B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatrica 2004;93(2):174-6. [PMID: ] - PubMed
Tanganelli 1992 {published data only}
-
- Regesta G, Tanganelli P. The risk of malformations and developmental disturbances in children exposed to antiepileptic drugs: a prospective controlled study. Bollettino Lega Italiana contro l'Epilessia 1996;95/96:351-4.
-
- Tanganelli P, Regesta G. Epilepsy, pregnancy, and major birth anomalies: an Italian prospective, controlled study. Neurology 1992;42(4 Suppl 5):89-93. [PMID: ] - PubMed
UK and Ireland Epilepsy and Pregnancy Register {published and unpublished data}
-
- Campbell E, Devenney E, Morrow J, Russell A, Smithson WH, Parsons L, et al. Recurrence risk of congenital malformations in infants exposed to antiepileptic drugs in utero. Epilepsia 2013;54(1):165-71. [PMID: ] - PubMed
-
- Campbell E, Kennedy F, Russell A, Smithson WH, Parsons L, Morrison PJ, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. Journal of Neurology, Neurosurgery & Psychiatry 2014;85(9):1029-34. [PMID: ] - PubMed
-
- Hunt S, Craig J, Russell A, Guthrie E, Parsons L, Robertson I, et al. Levetiracetam in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 2006;67(10):1876-9. [PMID: ] - PubMed
-
- Hunt S, Russell A, Smithson WH, Parsons L, Robertson I, Waddell R, et al. Topiramate in pregnancy. Neurology 2008;71(4):272-6. [PMID: ] - PubMed
-
- Kinney MO, Morrow J, Patterson CC, Campbell E, Russell A, Smithson HW, et al. Changing antiepilepsy drug-prescribing trends in women with epilepsy in the UK and Ireland and the impact on major congenital malformations. Journal of Neurology, Neurosurgery, and Psychiatry 2018;89(12):1320-3. [PMID: ] - PubMed
UK Clinical Research Practice Database {published data only}
-
- Charlton RA, Weil JG, Cunnington MC, De Vries CS. Identifying major congenital malformations in the UK General Practice Research Database (GPRD): a study reporting on the sensitivity and added value of photocopied medical records and free text in the GPRD. Drug Safety 2010;33(9):741-50. [PMID: ] - PubMed
-
- Charlton RA, Weil JG, Cunnington MC, Ray S, De Vries CS. Comparing the General Practice Research Database and the UK Epilepsy and Pregnancy Register as tools for postmarketing teratogen surveillance: anticonvulsants and the risk of major congenital malformations. Drug Safety 2011;34(2):157-71. [PMID: ] - PubMed
UK Health Record THIN Register {published data only}
US Medicaid Registers {published data only}
-
- Patorno E, Hernandez-Diaz S, Huybrechts KF, Desai RJ, Cohen JM, Mogun H, et al. Gabapentin in pregnancy and the risk of adverse neonatal and maternal outcomes: a population-based cohort study nested in the US Medicaid Analytic eXtract dataset. PLOS Medicine 2020;17(9):e1003322. [PMID: ] - PMC - PubMed
Waters 1994 {published data only}
-
- Waters CH, Belai Y, Gott PS, Shen P, De Giorgio CM. Outcomes of pregnancy associated with antiepileptic drugs. Archives of Neurology 1994;51(3):250-3. [PMID: ] - PubMed
References to studies excluded from this review
Annegers 1974 {published data only}
-
- Annegers JF, Elveback LR, Hauser WA, Kurland LT. Do anticonvulsants have a teratogenic effect? Archives of Neurology 1974;31(6):364-73. [PMID: ] - PubMed
-
- Annegers JF, Elveback LR, Hauser WA, Kurland LT. Epilepsy anticonvulsants and malformations. Birth Defects original article series 1975;11(5):157-60. [PMID: ] - PubMed
Arteaga‐Vazques 2012 {published data only}
-
- Arteaga-Vazquez J, Luna-Munoz L, Mutchinick OM. Congenital malformations in the offspring of epileptic mothers with and without anticonvulsant treatment [Malformaciones congenitas en hijos de madres epilepticas con y sin tratmiento con anticonvulsivantes]. Salud Publica Mexico 2012;54(6):579-86. [PMID: ] - PubMed
Arulmozhi 2006 {published data only}
-
- Arulmozhi T, Dhanaraj M, Rangaraj R, Vengatesan A. Physical growth and psychomotor development of infants exposed to antiepileptic drugs in utero. Neurology India 2006;54(1):42-6; discussion 47. [PMID: ] - PubMed
Baermig 1973 {published data only}
-
- Bärmig H. Epilepsy and pregnancy [Epilepsie und schwangerschaft]. Geburtshilfe und Frauenheilkunde 1973;33(3):203-4. [PMID: ] - PubMed
Borthen 2009 {published data only}
-
- Borthen I, Eide MG, Veiby G, Daltveit AK, Gilhus NE. Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG 2009;116(13):1736-42. [PMID: ] - PubMed
Bozhinov 2009 {published data only}
-
- Bozhinov P, Bozhinova C, Markova C. [Fetal malformations in women with epilepsy]. Akusherstvo i Ginekologiia 2009;48(1):16-21. [PMID: ] - PubMed
-
- Bozhinova S, Bozhinov P. The course of pregnancy and labor in patients with epilepsy [Protichane na bremennostta i razhdaneto pri bremenni s epilepsiia]. Akusherstvo i Ginekologiia 1998;37(4):12-4. [PMID: ] - PubMed
Canun‐Serrano 1986 {published data only}
-
- Canún-Serrano S, Zafra de la Rosa G, Landeros-Velázquez G, Givaudan-Moreno M. Anticonvulsants and pregnancy [Anticonvulsivos y embarazo]. Boletín Médico del Hospital Infantil de México 1986;43(4):219-27. [PMID: ] - PubMed
Castilla‐Puentes 2014 {published data only}
-
- Castilla-Puentes R, Ford L, Manera L, Kwarta RF Jr, Ascher S, Li Q. Topiramate monotherapy use in women with and without epilepsy: pregnancy and neonatal outcomes. Epilepsy Research 2014;108(4):717-24. [PMID: ] - PubMed
Diaz‐Romero 1999 {published data only}
-
- Díaz-Romero RM, Garza-Morales S, Mayén-Molina DG, Ibarra-Puig J, Avila-Rosas H. Facial anthropometric measurements in offspring of epileptic mothers. Archives of Medical Research 1999;30(3):186–9. [PMID: ] - PubMed
Dobos 1985 {published data only}
-
- Dobos M, Schuler D, Marosfi S, Bogáthy B. Congenital developmental anomalies in the offspring of epileptic mothers [Veleszületett fejlödési rendellenességek vizsgálata epilepsziás anyák utódaiban]. Orvosi Hetilap 1985;126(37):2267-72. [PMID: ] - PubMed
Dravet 1992 {published data only}
-
- Dravet C, Julian C, Legras C, Magaudda A, Guerrini R, Genton P, et al. Epilepsy, antiepileptic drugs, and malformations in children of women with epilepsy: a French prospective cohort study. Neurology 1992;42(4 Suppl 5):75-82. [PMID: ] - PubMed
Elshove 1971 {published data only}
-
- Elshove J, Van Eck JH. Congenital abnormalities, cleft lip and cleft palate in particular, in children of epileptic mothers [Aangeboren misvormingen, met name gespleten lip met of zonder gespleten verhemelte, bij kinderen van moeders met epilepsie]. Nederlands Tijdschrift voor Geneeskunde 1971;115(33):1371-5. [PMID: ] - PubMed
EMPiRE Study {published data only}
-
- Thangaratinam S, Marlin N, Newton S, Weckesser A, Bagary M, Greenhill L, et al. AntiEpileptic drug Monitoring in PREgnancy (EMPiRE): a double-blind randomised trial on effectiveness and acceptability of monitoring strategies. Southampton (UK): NIHR Journals Library (Health Technology Assessment, No. 22.23), 2018. [DOI: 10.3310/hta22230] [PMID: ] - DOI - PMC - PubMed
Finland Cohort Study {published data only}
-
- Gaily E, Granström ML, Hiilesmaa V, Bardy A. Minor anomalies in offspring of epileptic mothers. Journal of Pediatrics 1988;112(4):520-9. [PMID: ] - PubMed
-
- Gaily E. Distal phalangeal hypoplasia in children with prenatal phenytoin exposure: results of a controlled anthropometric study. American Journal of Medical Genetics 1990;35(4):574-8. [PMID: ] - PubMed
-
- Gaily EK, Granström ML, Hiilesmaa VK, Bardy AH. Head circumference in children of epileptic mothers: contributions of drug exposure and genetic background. Epilepsy Research 1990;5(3):217-22. [PMID: ] - PubMed
-
- Hiilesmaa VK, Bardy A, Teramo K. Obstetric outcome in women with epilepsy. American Journal of Obstetrics and Gynecology 1985;152(5):499-504. [PMID: ] - PubMed
Fujji 2013 {published data only}
Galappatty 2018 {published data only}
-
- Galappatthy P, Liyanage CK, Lucas MN, Jayasekara DTLM, Abhayaratna SA, Weeraratne C, et al. Obstetric outcomes and effects on babies born to women treated for epilepsy during pregnancy in a resource limited setting: a comparative cohort study. BMC Pregnancy & Childbirth 2018;18(1):230. [PMID: ] - PMC - PubMed
Goujard 1974 {published data only}
-
- Goujard J, Huel G, Rumeau-Rouquette C. Antiepileptics and congenital malformations [Antiepileptiques et malformations congenitales]. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction 1974;3(6):831-42. [PMID: ] - PubMed
Hill 1974 {published data only}
-
- Hill RM, Verniaud WM, Horning MG, McCulley LB, Morgan NF. Infants exposed in utero to antiepileptic drugs: a prospective study. American Journal of Diseases of Children 1974;127(5):645-53. [PMID: ] - PubMed
Holmes 1994 {published data only}
-
- Holmes LB, Harvey EA, Brown KS, Hayes AM, Khoshbin S. Anticonvulsant teratogenesis: 1. A study design for newborn infants. Teratology 1994;49(3):202-7. [PMID: ] - PubMed
Jacobsen 2014 {published data only}
Jedrzejczak 2022 {published data only}
-
- Jedrzejczak J, Majkowska-Zwolinska B. Clinical predictors for breastfeeding initiation among women with epilepsy. Seizure 2022;96:59-65. [PMID: ] - PubMed
Jones 1989 {published data only}
-
- Jones KL, Lacro RV, Johnson KA, Adams J. Pattern of malformations in the children of women treated with carbamazepine during pregnancy. New England Journal of Medicine 1989;320(25):1661-6. [PMID: ] - PubMed
Knight 1975 {published data only}
-
- Knight AH, Rhind EG. Epilepsy and pregnancy: a study of 153 pregnancies in 59 patients. Epilepsia 1975;16(1):99-110. [PMID: ] - PubMed
Lamotrigine Pregnancy Registry {published data only}
-
- Cunnington M, Ferber S, Quartey G, International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Effect of dose on the frequency of major birth defects following fetal exposure to lamotrigine monotherapy in an international observational study. Epilepsia 2007;48(6):1207-10. [PMID: ] - PubMed
-
- Cunnington MC. The International Lamotrigine Pregnancy Registry update for the Epilepsy Foundation. Epilepsia 2004;45(11):1468. [PMID: ] - PubMed
-
- Lamotrigine Pregnancy Registry. Final report: 1 September 1992–31 March 2010. pregnancyregistry.gsk.com/documents/lam_spring_2010_final_report.pdf 2010 (accessed prior to 4 Feb 2023).
-
- Tennis P, Eldridge RR, International Lamotrigine Pregnancy Registry Scientific Advisory Committee. Preliminary results on pregnancy outcomes in women using lamotrigine. Epilepsia 2002;43(10):1161-7. [PMID: ] - PubMed
Laskowska 2002 {published data only}
-
- Laskowska M, Leszczyńska-Gorzelak B, Oleszczuk J. Evaluation of antiepileptic therapy during pregnancy [Ocena terapii przeciwpadaczkowej w okresie ciazy]. Ginekologia Polska 2002;73(1):35-42. [PMID: ] - PubMed
Miskov 2009 {published data only}
-
- Miskov S, Gjergja-Juraski R, Cvitanovic-Sojat L, Bakulic TI, Fucic A, Bosnjak-Pasic M, et al. Prospective surveillance of Croatian pregnant women on lamotrigine monotherapy - aspects of pre-pregnancy counseling and drug monitoring. Acta Clinica Croatica 2009;48(3):271-81. [PMID: ] - PubMed
Monson 1973 {published data only}
-
- Monson RR, Rosenberg L, Hartz SC, Shapiro S, Heinonen OP, Slone D. Diphenylhydantoin and selected congenital malformations. New England Journal of Medicine 1973;289(20):1049-52. [PMID: ] - PubMed
Montouris 2003 {published data only}
-
- Montouris G. Gabapentin exposure in human pregnancy: results from the Gabapentin Pregnancy Registry. Epilepsy & Behavior 2003;4(3):310-7. [PMID: ] - PubMed
Mostacci 2018 {published data only}
-
- Mostacci B, Bisulli F, Poluzzi E, Cocchi G, Piccinni C, Curti A, et al. Emilia-Romagna Study on Pregnancy and Exposure to Antiepileptic drugs (ESPEA): a population-based study on prescription patterns, pregnancy outcomes and fetal health. Journal of Neurology, Neurosurgery & Psychiatry 2018;89(9):983-8. [PMID: ] - PMC - PubMed
-
- Mostacci B, Piccinni C, Bisulli F, Poluzzi E, Naldi I, Accetta G, et al. Prevalence of antiepileptic drugs exposure in pregnant women in the Emilia Romagna region (Italy): results from the ESPEA (Emilia Romagna Study on Pregnancy and Exposure to Antiepileptic Drugs). Epilepsia 2014;55(Suppl 2):131-2, Abstract no: p400.
Nakane 1980 {published data only}
-
- Murasaki O, Yoshitake K, Tachiki H, Nakane Y, Kaneko S. Reexamination of the teratological effect of antiepileptic drugs. Japanese Journal of Psychiatry and Neurology 1988;42(3):592-3. [PMID: ] - PubMed
-
- Nakane Y, Okuma T, Takahashi R, Sato Y, Wada T, Sato T, et al. Multi-institutional study on the teratogenicity and fetal toxicity of antiepileptic drugs: a report of a collaborative study group in Japan. Epilepsia 1980;21(6):663-80. [PMID: ] - PubMed
-
- Nakane Y. Congenital malformation among infants of epileptic mothers treated during pregnancy: the report of a collaborative study group in Japan. Folia Psychiatrica et Neurologica Japonica 1979;33(3):363-9. [PMID: ] - PubMed
Pearse 1992 {published data only}
-
- Pearse SB, Garcia Rodriguez LA, Hartwell C, Russell G. A pregnancy register of patients receiving carbamazepine in the UK. Pharmacoepidemiology and Drug Safety 1992;1(6):321-5. [DOI: 10.1002/pds.2630010603] - DOI
Richmond 2004 {published data only}
-
- Richmond JR, Krishnamoorthy P, Andermann E, Benjamin A. Epilepsy and pregnancy: an obstetric perspective. American Journal of Obstetrics and Gynecology 2004;190(2):371-9. [PMID: ] - PubMed
Robert 1983 {published data only}
-
- Robert E, Robert JM, Lapras C. Is valproic acid teratogenic? [L'acide valproïque est-il tératogène?]. Revue Neurologique 1983;139(6-7):445-7. [PMID: ] - PubMed
Sabers 2004 {published data only}
-
- Sabers A, Dam M, A-Rogvi-Hansen B, Boas J, Sidenius P, Laue Friis M, et al. Epilepsy and pregnancy: lamotrigine as main drug used. Acta Neurologica Scandinavica 2004;109(1):9-13. - PubMed
Scheuerle 2019 {published data only}
-
- Scheuerle AE, Holmes LB, Albano JD, Badalamenti V, Battino D, Covington D, et al. Levetiracetam Pregnancy Registry: final results and a review of the impact of registry methodology and definitions on the prevalence of major congenital malformations. Birth Defects Research 2019;111(13):872-87. [PMID: ] - PubMed
Shapiro 1976 {published data only}
-
- Shapiro S, Hartz SC, Siskind V, Mitchell AA, Slone D, Rosenberg L, et al. Anticonvulsants and parental epilepsy in the development of birth defects. Lancet 1976;1(7954):272-5. [PMID: ] - PubMed
Starveld‐Zimmerman 1975 {published data only}
-
- Starveld-Zimmerman A, Van der Kolk W, Elshove J, Meinardi H. Teratogenicity of antiepileptic drugs. Clinical Neurology and Neurosurgery 1975;77(2):81-95. [PMID: ] - PubMed
Tennis 2015 {published data only}
-
- Tennis P, Chan KA, Curkendall SM, Li D-K, Mines D, Peterson C, et al. Topiramate use during pregnancy and major congenital malformations in multiple populations. Birth Defects Research 2015;103(4):269-75. [PMID: ] - PubMed
Torres 1995 {published data only}
-
- Torres LC, Félix R, Canún S, Mazón JJ. Epilepsy and pregnancy: risks and benefits of anticonvulsant treatments [Epilepsia y embarazo. Riegos y beneficios del tratamiento anticonvulsivo]. Ginecologia y Obstetricia de Mexico 1995;63:282-6. [PMID: ] - PubMed
Wide 2000 {published data only}
-
- Wide K, Winbladh B, Tomson T, Sars-Zimmer K, Berggren E. Psychomotor development and minor anomalies in children exposed to antiepileptic drugs in utero: a prospective population-based study. Developmental Medicine and Child Neurology 2000;42(2):87-92. [PMID: ] - PubMed
Yeh 2017 {published data only}
Yerby 1992 {published data only}
-
- Yerby MS, Leavitt A, Erickson DM, McCormick KB, Loewenson RB, Sells CJ, et al. Antiepileptics and the development of congenital anomalies. Neurology 1992;42(4 Suppl 5):132-40. [PMID: ] - PubMed
References to studies awaiting assessment
Babic 2014 {published data only}
-
- Babic N, Jovic M. Postnatal concerns in children born to women with juvenile myoclonic epilepsy. Epilepsia 2014;55(Suppl 2):128, Abstract no: p389.
Kaabi 2013 {published data only}
-
- Kaabi W, El Aidli S, Kastalli S, Lakhoua G, Zaiem A, Srairi S, et al. Pregnancy outcomes in women using antiepileptic drugs. Drug Safety 2013;36(9):844, Abstract no: ISP3556-47.
Kutlu 2013 {published data only}
-
- Kutlu G, Erdal A, Aydogan S, Gomceli YB, Inan LE. Follow up and treatment of women with epilepsy during pregnancy. Epilepsia 2013;54(Suppl 3):127-8, Abstract no: P399.
Lazzaroni Fossati 1986 {published data only}
-
- Lazzaroni Fossati F, De Toni T, Magnani M, Repetto E, Calvi A, Di Siena G. Intrauterine exposure to drugs: analysis of a sample of newborn infants pretreated with anticonvulsants [Esposizione in utero a farmaci. Analisi di un campione di neonati pretrattati con anticonvulsivanti]. Minerva Pediatrica 1986;38(3-4):75-81. [PMID: ] - PubMed
Midi 2014 {published data only}
-
- Midi I, Bulut B, Ozbek D, Ozden HO, Agan K. Antiepileptic drug usage and the effects of them on the foetus in epileptic pregnant woman. Epilepsia 2014;55(Suppl 2):132, Abstract no: p401.
-
- Midi I, Cetinkaya DO, Ozden HO, Agan K. Pregnant women with epilepsy: 43 patients results in 1 year period. Epilepsia 2013;54(Suppl 3):131, Abstract no: P410.
Shvartzman 1986 {published data only}
-
- Shvartzman P, Oren B, Keinan A, Adar H. [Congenital malformations and anticonvulsant therapy in pregnancy]. Harefuah 1986;110(8):377-80. [PMID: ] - PubMed
Vlasov 2014 {published data only}
-
- Vlasov P, Petrukhin V, Karlov V, Krasnopolski V, Melnikov A, Tsivtsivadze E. Antiepileptic drug therapy during pregnancy and obstetric outcomes in Moscow region: comparing of 1998 and 2013 years. Epilepsia 2014;55(Suppl 2):130, Abstract no: p396.
Additional references
Ackers 2009
-
- Ackers R, Besag FM, Wade A, Murray ML, Wong IC. Changing trends in antiepileptic drug prescribing in girls of child-bearing potential. Archives of Disease in Childhood 2009;94(6):443–7. [PMID: ] - PubMed
Alsaad 2015
-
- Alsaad AM, Chaudhry SA, Koren G. First trimester exposure to topiramate and the risk of oral clefts in the offspring: a systematic review and meta-analysis. Reproductive Toxicology 2015;53:45-50. [PMID: ] - PubMed
Ardinger 1988
-
- Ardinger HH, Atkin JF, Blackston RD, Elsas LJ, Clarren SK, Livingstone S, et al. Verification of the fetal valproate syndrome phenotype. American Journal of Medical Genetics 1988;29(1):171-85. [PMID: ] - PubMed
Brent 2004
-
- Brent RL. Environmental causes of human congenital malformations: the pediatrician's role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics 2004;113(4 Suppl):957-68. [PMID: ] - PubMed
Bromley 2014
Charlton 2008
-
- Charlton RA, Cunnington MC, De Vries CS, Weil JG. Data resources for investigating drug exposure during pregnancy and associated outcomes: the General Practice Research Database (GPRD) as an alternative to pregnancy registries. Drug Safety 2008;31(1):39-51. [PMID: ] - PubMed
Chaudhry 2014
-
- Chaudhry SA, Jong G, Koren G. The fetal safety of levetiracetam: a systematic review. Reproductive Toxicology 2014;46:40-5. [PMID: ] - PubMed
Christensen 2013
Christensen 2019
Clayton‐Smith 2019
-
- Clayton-Smith J, Bromley R, Dean J, Journel H, Odent S, Wood A, et al. Diagnosis and management of individuals with Fetal Valproate Spectrum Disorder; a consensus statement from the European Reference Network for Congenital Malformations and Intellectual Disability. Orphanet Journal of Rare Diseases 2019;14(1):180. [PMID: ] - PMC - PubMed
Dean 2000
-
- Dean JC, Moore SJ, Turnpenny PD. Developing diagnostic criteria for the fetal anticonvulsant syndromes. Seizure 2000;9(3):233–4. [PMID: ] - PubMed
DiLiberti 1984
-
- DiLiberti JH, Farndon PA, Dennis NR, Curry CJ. The fetal valproate syndrome. American Journal of Medical Genetics 1984;19(3):473-81. [PMID: ] - PubMed
EUROCAT
-
- EUROCAT European Surveillance of Congenital Anomalies. EUROCAT guide 1.3 and reference documents: instructions for the registration and surveillance of congenital anomalies. eu-rd-platform.jrc.ec.europa.eu/sites/default/files/EUROCAT-Guide-1.3.pdf (accessed August 2015).
Fiest 2017
Guyatt 2008
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Jentink 2010a
-
- Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. New England Journal of Medicine 2010;362(23):2185-93. [PMID: ] - PubMed
Jentink 2010b
Ku 2011
-
- Ku CS, Naidoo N, Wu M, Soong R. Studying the epigenome using next generation sequencing. Journal of Medical Genetics 2011;48(11):721-30. [PMID: ] - PubMed
Man 2012
Margulis 2012
Marson 2007
-
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al, SANAD Study group. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 2007;369(9566):1016-26. [PMID: ] - PMC - PubMed
Meador 2008
Meador 2009
Mines 2014
-
- Mines D, Tennis P, Curkendall SM, Li DK, Peterson C, Andrews EB, et al. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiology and Drug Safety 2014;23(10):1017–25. [PMID: ] - PubMed
NICE 2022
-
- National Institute for Clinical Excellence (NICE). Epilepsies in children, young people and adults. NICE guideline [NG217]. www.nice.org.uk/guidance/ng217 (accessed prior to 4 Feb 2023).
Oommen 1999
-
- Oommen KJ, Mathews S. Zonisamide: a new antiepileptic drug. Clinical Neuropharmacology 1999;22(4):192-200. [PMID: ] - PubMed
Sterne 2016
Tetro 2017
-
- Tetro N, Moushaev S, Shmuel M, Eyal S. Antiseizure medications and fetal nutrients: effects on choline transporters in a human placental cell line. Epilepsia 2021;62(6):1451-9. [PMID: ] - PubMed
Tomson 2011
-
- Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al, EURAP study group. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurology 2011;10(7):609-17. [PMID: ] - PubMed
Tomson 2015
-
- Tomson T, Marson A, Boon P, Canevini MP, Covanis A, Gaily E, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia 2015;56(7):1006-19. [PMID: ] - PubMed
Veroniki 2017
References to other published versions of this review
Adab 2004
Pulman 2012
Weston 2016
-
- Weston J, Bromley R, Jackson CF, Adab N, Clayton-Smith J, Greenhalgh J, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD010224. [DOI: 10.1002/14651858.CD010224.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

