Timing of dense granule biogenesis in asexual malaria parasites
- PMID: 37647112
- PMCID: PMC10482371
- DOI: 10.1099/mic.0.001389
Timing of dense granule biogenesis in asexual malaria parasites
Abstract
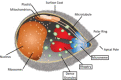
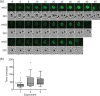
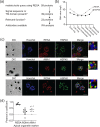
Malaria is an important infectious disease that continues to claim hundreds of thousands of lives annually. The disease is caused by infection of host erythrocytes by apicomplexan parasites of the genus Plasmodium. The parasite contains three different apical organelles - micronemes, rhoptries and dense granules (DGs) - whose contents are secreted to mediate binding to and invasion of the host cell and the extensive remodelling of the host cell that occurs following invasion. Whereas the roles of micronemes and rhoptries in binding and invasion of the host erythrocyte have been studied in detail, the roles of DGs in Plasmodium parasites are poorly understood. They have been proposed to control host cell remodelling through regulated protein secretion after invasion, but many basic aspects of the biology of DGs remain unknown. Here we describe DG biogenesis timing for the first time, using RESA localization as a proxy for the timing of DG formation. We show that DG formation commences approximately 37 min prior to schizont egress, as measured by the recruitment of the DG marker RESA. Furthermore, using a bioinformatics approach, we aimed to predict additional cargo of the DGs and identified the J-dot protein HSP40 as a DG protein, further supporting the very early role of these organelles in the interaction of the parasite with the host cell.
Keywords: Plasmodium; apical organelles; apicomplexa; dense granules; malaria.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

