Novel Biomaterial Containing Gelatin, Manuka Honey, and Hydroxyapatite Enhanced Secondary Intention Healing Versus Standard Secondary Intention Healing in Mohs Surgical Defects on the Head and Distal Lower Extremities-A Randomized Controlled Trial: Pilot Study
- PMID: 37647156
- PMCID: PMC11111313
- DOI: 10.1097/DSS.0000000000003924
Novel Biomaterial Containing Gelatin, Manuka Honey, and Hydroxyapatite Enhanced Secondary Intention Healing Versus Standard Secondary Intention Healing in Mohs Surgical Defects on the Head and Distal Lower Extremities-A Randomized Controlled Trial: Pilot Study
Abstract
Background: Randomized, comparative studies evaluating augmented secondary intention healing (SIH) compared with conventional SIH in dermatologic surgery are limited. This study aimed to evaluate whether the use of a novel biomaterial enhances SIH, particularly in shortening time to complete re-epithelialization.
Objective: The purpose of this study was to elucidate whether a novel biomaterial containing gelatin, manuka honey, and hydroxyapatite enhances SIH when compared with conventional SIH for surgical defects after Mohs micrographic surgery (MMS) on the head and distal lower extremities.
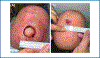
Materials and methods: Thirty-seven patients were enrolled in this randomized controlled trial. Patients undergoing MMS on the head or distal lower extremities were eligible for recruitment. After clear surgical margins were obtained post-MMS, patients were randomized to receive standard SIH or biomaterial enhanced SIH. Patients had regularly scheduled follow-ups with questionnaires at each visit until complete re-epithelialization was achieved.
Results: Overall, there was no significant difference in time to re-epithelialization between standard SIH and biomaterial-enhanced SIH. However, there was a significant decrease in pain scores and skin thickness in the biomaterial-enhanced SIH group.
Conclusion: Biomaterial-enhanced SIH is noninferior to standard SIH and produces less pain and favorable skin thickness compared with standard SIH. ClinicalTrials.gov listing: NCT04545476.
Copyright © 2023 by the American Society for Dermatologic Surgery, Inc. Published by Wolters Kluwer Health, Inc. All rights reserved.
Figures


References
-
- Zitelli JA. Wound healing by secondary intention. A cosmetic appraisal. J Am Acad Dermatol 1983;9:407–15. - PubMed
-
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol 1984;2:92–106. - PubMed
-
- Donaldson MR, Coldiron BM. Scars after second intention healing.Facial Plast Surg 2012;28:497–503. - PubMed
-
- Stebbins WG, Gusev J, Higgins HW II, Nelson A, et al. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol 2015;73:865–7.e1. - PubMed
-
- Chern PL, Baum CL, Arpey CJ. Biologic dressings: current applications and limitations in dermatologic surgery. Dermatol Surg 2009;35:891–906. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

