Immunosequencing and Profiling of T Cells at the Maternal-Fetal Interface of Women with Preterm Labor and Chronic Chorioamnionitis
- PMID: 37647360
- PMCID: PMC10528178
- DOI: 10.4049/jimmunol.2300201
Immunosequencing and Profiling of T Cells at the Maternal-Fetal Interface of Women with Preterm Labor and Chronic Chorioamnionitis
Abstract
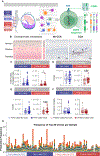
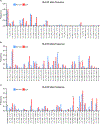
T cells are implicated in the pathophysiology of preterm labor and birth, the leading cause of neonatal morbidity and mortality worldwide. Specifically, maternal decidual T cells infiltrate the chorioamniotic membranes in chronic chorioamnionitis (CCA), a placental lesion considered to reflect maternal anti-fetal rejection, leading to preterm labor and birth. However, the phenotype and TCR repertoire of decidual T cells in women with preterm labor and CCA have not been investigated. In this study, we used phenotyping, TCR sequencing, and functional assays to elucidate the molecular characteristics and Ag specificity of T cells infiltrating the chorioamniotic membranes in women with CCA who underwent term or preterm labor. Phenotyping indicated distinct enrichment of human decidual effector memory T cell subsets in cases of preterm labor with CCA without altered regulatory T cell proportions. TCR sequencing revealed that the T cell repertoire of CCA is characterized by increased TCR richness and decreased clonal expansion in women with preterm labor. We identified 15 clones associated with CCA and compared these against established TCR databases, reporting that infiltrating T cells may possess specificity for maternal and fetal Ags, but not common viral Ags. Functional assays demonstrated that choriodecidual T cells can respond to maternal and fetal Ags. Collectively, our findings provide, to our knowledge, novel insight into the complex processes underlying chronic placental inflammation and further support a role for effector T cells in the mechanisms of disease for preterm labor and birth. Moreover, this work further strengthens the contribution of adaptive immunity to the syndromic nature of preterm labor and birth.
Copyright © 2023 by The American Association of Immunologists, Inc.
Conflict of interest statement
DISCLOSURE
The authors have no financial conflicts of interest.
Figures






Similar articles
-
Are B cells altered in the decidua of women with preterm or term labor?Am J Reprod Immunol. 2019 May;81(5):e13102. doi: 10.1111/aji.13102. Epub 2019 Mar 29. Am J Reprod Immunol. 2019. PMID: 30768818 Free PMC article.
-
Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes.Am J Obstet Gynecol. 2017 Nov;217(5):592.e1-592.e17. doi: 10.1016/j.ajog.2017.08.008. Epub 2017 Aug 25. Am J Obstet Gynecol. 2017. PMID: 28847437 Free PMC article.
-
Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor.J Immunol Res. 2019 May 23;2019:3128010. doi: 10.1155/2019/3128010. eCollection 2019. J Immunol Res. 2019. PMID: 31263712 Free PMC article.
-
Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance.Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S53-69. doi: 10.1016/j.ajog.2015.08.041. Am J Obstet Gynecol. 2015. PMID: 26428503 Free PMC article. Review.
-
Maternal and fetal T cells in term pregnancy and preterm labor.Cell Mol Immunol. 2020 Jul;17(7):693-704. doi: 10.1038/s41423-020-0471-2. Epub 2020 May 28. Cell Mol Immunol. 2020. PMID: 32467619 Free PMC article. Review.
Cited by
-
Immunophenotyping and Activation Status of Maternal Lymphocytes to Predict Spontaneous Preterm Birth in Women With Threatened Preterm Labor: A Prospective Observational Study.Am J Reprod Immunol. 2024 Dec;92(6):e70015. doi: 10.1111/aji.70015. Am J Reprod Immunol. 2024. PMID: 39625044 Free PMC article.
-
Progestogen-driven B7-H4 contributes to onco-fetal immune tolerance.Cell. 2024 Aug 22;187(17):4713-4732.e19. doi: 10.1016/j.cell.2024.06.012. Epub 2024 Jul 4. Cell. 2024. PMID: 38968937 Free PMC article.
References
-
- Aluvihare VR, Kallikourdis M, and Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5: 266–271. - PubMed
-
- Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, and Volk HD. 2005. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 166: 811–822. - PMC - PubMed
-
- Robertson SA, Guerin LR, Moldenhauer LM, and Hayball JD. 2009. Activating T regulatory cells for tolerance in early pregnancy - the contribution of seminal fluid. J Reprod Immunol 83: 109–116. - PubMed
-
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, and Saito S. 2010. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol 85: 121–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

