The anti-CD40L monoclonal antibody AT-1501 promotes islet and kidney allograft survival and function in nonhuman primates
- PMID: 37647390
- PMCID: PMC10990482
- DOI: 10.1126/scitranslmed.adf6376
The anti-CD40L monoclonal antibody AT-1501 promotes islet and kidney allograft survival and function in nonhuman primates
Abstract
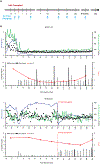
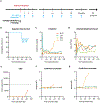
Prior studies of anti-CD40 ligand (CD40L)-based immunosuppression demonstrated effective prevention of islet and kidney allograft rejection in nonhuman primate models; however, clinical development was halted because of thromboembolic complications. An anti-CD40L-specific monoclonal antibody, AT-1501 (Tegoprubart), was engineered to minimize risk of thromboembolic complications by reducing binding to Fcγ receptors expressed on platelets while preserving binding to CD40L. AT-1501 was tested in both a cynomolgus macaque model of intrahepatic islet allotransplantation and a rhesus macaque model of kidney allotransplantation. AT-1501 monotherapy led to long-term graft survival in both islet and kidney transplant models, confirming its immunosuppressive potential. Furthermore, AT-1501-based regimens after islet transplant resulted in higher C-peptide, greater appetite leading to weight gain, and reduced occurrence of cytomegalovirus reactivation compared with conventional immunosuppression. These data support AT-1501 as a safe and effective agent to promote both islet and kidney allograft survival and function in nonhuman primate models, warranting further testing in clinical trials.
Figures


References
-
- Vincenti F, Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol 121, 299–306; quiz 307-298 (2008). - PubMed
-
- Archdeacon P, Dixon C, Belen O, Albrecht R, Meyer J, Summary of the US FDA approval of belatacept. Am J Transplant 12, 554–562 (2012). - PubMed
-
- Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP, Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 374, 333–343 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

