Structures of wild-type and selected CMT1X mutant connexin 32 gap junction channels and hemichannels
- PMID: 37647412
- PMCID: PMC10468125
- DOI: 10.1126/sciadv.adh4890
Structures of wild-type and selected CMT1X mutant connexin 32 gap junction channels and hemichannels
Abstract
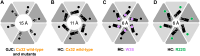
In myelinating Schwann cells, connection between myelin layers is mediated by gap junction channels (GJCs) formed by docked connexin 32 (Cx32) hemichannels (HCs). Mutations in Cx32 cause the X-linked Charcot-Marie-Tooth disease (CMT1X), a degenerative neuropathy without a cure. A molecular link between Cx32 dysfunction and CMT1X pathogenesis is still missing. Here, we describe the high-resolution cryo-electron cryo-myography (cryo-EM) structures of the Cx32 GJC and HC, along with two CMT1X-linked mutants, W3S and R22G. While the structures of wild-type and mutant GJCs are virtually identical, the HCs show a major difference: In the W3S and R22G mutant HCs, the amino-terminal gating helix partially occludes the pore, consistent with a diminished HC activity. Our results suggest that HC dysfunction may be involved in the pathogenesis of CMT1X.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

