Therapeutic strategies and promising vaccine for hepatitis C virus infection
- PMID: 37647422
- PMCID: PMC10461427
- DOI: 10.1002/iid3.977
Therapeutic strategies and promising vaccine for hepatitis C virus infection
Abstract
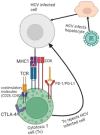
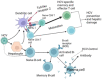
Hepatitis C virus (HCV) infection is still a significant global health problem despite therapeutic advancements. Ribavirin and interferon therapy have been the sole available treatments for HCV infection for a number of years with low efficacy. Thus, currently, a number of therapeutic strategies are being used, including nanoparticles (NPs), micro-RNAs such as small interfering RNA (siRNA), RNAi-based gene silencing and antisense oligonucleotide-based microRNA-122, microRNA-155, and short hairpin RNAs (shRNAs), and immunotherapeutic approaches such as anti-programmed cell death 1(PD-1), monoclonal antibodies (mAb or moAb), and monocyte-derived dendritic cells (Mo-DCs). Furthermore, direct-acting antivirals (DAAs) and host-targeting agents (HTA) were also the current therapeutic approaches with great efficacy. In spite of different clinical trials on HCV vaccine developments, nowadays there is no effective HCV vaccine in opposition to virus due to various challenges including genetic diversity, lack of immunocompetent small animal models, shortage of HCV vaccination testing alternatives, lack of an effective tissue culture method for replicating HCV, and inadequate knowledge regarding to immune responses against HCV infection. Nowadays, mRNA vaccine, recombinant viral vector, peptides vaccine, virus-like particles, DNA vaccine, rational designed vaccine, and recombinant polyantigenic T-cell-based vaccine are novel promising candidates for HCV vaccine based on various clinical trials. This review summarizes the different therapeutic approaches and the advancements of vaccine candidates for HCV infection.
Keywords: hepatitis C virus; promising vaccine; therapeutic strategies.
© 2023 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett's principles and practice of infectious diseases E‐book. Elsevier Health Sciences; 2019.
-
- Butt F, Shahid M, Hassan M, et al. A review on hepatitis C virus: role of viral and host‐cellular factors in replication and existing therapeutic strategies. Egypt Liver J. 2022;12(1):71.
-
- Heim MH. 25 years of interferon‐based treatment of chronic hepatitis C: an epoch coming to an end. Nat Rev Immunol. 2013;13(7):535‐542. - PubMed
-
- Latief U, Tung GK, Per TS, et al. Micro RNAs as emerging therapeutic targets in liver diseases. Curr Protein Pept Sci. 2022;23(6):369‐383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

