Effects of N-acetyl-L-cysteine polysulfides on periodontitis in a mouse model
- PMID: 37647428
- PMCID: PMC10408371
- DOI: 10.1002/iid3.959
Effects of N-acetyl-L-cysteine polysulfides on periodontitis in a mouse model
Abstract
Background: Polysulfides are reported to be involved in various important biological processes. N-acetyl-l-cysteine polysulfide with 2 sulfane sulfur atoms (NAC-S2) regulates diverse toll-like receptor (TLR) signaling pathways. Here, we aimed to determine the role of NAC-S2 in periodontitis and explore the potential mechanism.
Methods: A periodontitis mouse model was established by ligating the subgingival between the first and second molars in wild-type, TLR4-/- , and Myd88-/- mice.
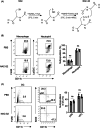
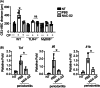
Results: NAC-S2 did not affect the proportion of macrophages (CD11b+ F4/80+ ) or neutrophils (CD11b+ GR-1+ ) in the bone marrow. Mechanically, lipopolysaccharides (LPS), Zymosan A, or poly I: C induced tumor necrosis factor (TNF), interleukin (IL)-6, and IL-1β expression in bone marrow-derived macrophages (BMDMs) could be inhibited by NAC-S2. On the other hand, NAC-S2 suppressed the phosphorylation levels of IκB-α, p65, and IκB kinase (IKK)-β induced by LPS in BMDMs, while LPS induced phosphorylation of ERK1/2, p38, and transforming growth factor β-activated kinase 1 (TAK1) could not be affected by NAC-S2. In wild-type periodontitis mice, NAC-S2 administration decreased the cemento-enamel-junction-alveolar bone crest (CEJ-ABC) distance and the relative mRNA expression of TNF, IL-6, and IL-1β, while such phenomena could not be observed in TLR4 deficiency or Myd88 deficiency mice.
Conclusions: All of these results indicate that NAC-S2 ameliorates TLR4/NF-κB pathway mediated inflammation in mouse periodontitis model.
Keywords: NAC-S2; NF-κB; TLR4; inflammation; periodontitis.
© 2023 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dempsey LA. Defective fibrinolysis in periodontitis. Nat Immunol. 2022;23:150. - PubMed
-
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. - PubMed
-
- Kumaar NR, Nair SC. Nanomaterials: an intra‐periodontal pocket drug‐delivery system for periodontitis. Ther Delivery. 2023;14:227‐249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

