E2F3 renders an immunosuppressive tumor microenvironment in nasopharyngeal carcinoma: Involvements of the transcription activation of PRC1 and BIRC5
- PMID: 37647439
- PMCID: PMC10461428
- DOI: 10.1002/iid3.987
E2F3 renders an immunosuppressive tumor microenvironment in nasopharyngeal carcinoma: Involvements of the transcription activation of PRC1 and BIRC5
Abstract
Background: E2F transcription factors are well-recognized oncogenic molecules, and their correlation with immune cell infiltration has recently been reported. This work studies the impacts and mechanism of E2F transcription factor 3 (E2F3) in the growth and tumor microenvironment (TME) of nasopharyngeal carcinoma (NPC).
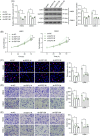
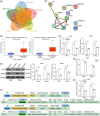
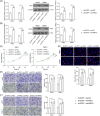
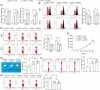
Methods: Aberrantly expressed transcription factors in NPC were screened by abundant bioinformatics analyses. Gene expression in NPC cells was analyzed by reverse transcription-quantitative polymerase chain reaction and Western blot analyses. Malignant behaviors of NPC cells were analyzed by cell counting kit-8, 5-ethynyl-2'-deoxyuridine labeling, Transwell assays, and xenograft tumor models. TPA-induced THP-1 cells (macrophages) were cultured in the conditioned medium of NPC cells to mimic tumor-associated macrophages (TAMs) in vivo, and these TAMs were cocultured with CD8+ T cells. Regulation of E2F3 on protein regulator of cytokinesis 1 (PRC1) and baculoviral IAP repeat containing 5 (BIRC5) was validated by chromatin immunoprecipitation and luciferase reporter assays.
Results: E2F3 was highly expressed in NPC cells, and its knockdown suppressed malignant behavior and tumorigenic ability of the cells. The E2F3 knockdown condition downregulated M2 cytokines CD163 and interleukin-10 in TAMs, which further enhanced proliferation and activation of the cocultured CD8+ T cells. E2F3 promoted transcription of PRC1 and BRIC5. Furthermore, PRC1 or BRIC5 upregulation in NPC cells restored the malignant properties of NPC cells, reprogrammed the TAMs to M2 phenotype, and suppressed the CD8+ T cell proliferation and activation.
Conclusion: This work suggests that E2F3 renders an immunosuppressive TME in NPC by activating PRC1 and BIRC5. Suppression of any member involved might favor tumor elimination.
Keywords: E2F transcription factor 3; baculoviral IAP repeat containing 5; macrophages; nasopharyngeal carcinoma; protein regulator of cytokinesis 1; tumor microenvironment.
© 2023 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A significant role of transcription factors E2F in inflammation and tumorigenesis of nasopharyngeal carcinoma.Biochem Biophys Res Commun. 2020 Apr 16;524(4):816-824. doi: 10.1016/j.bbrc.2020.01.158. Epub 2020 Feb 7. Biochem Biophys Res Commun. 2020. PMID: 32044038
-
MicroRNA-432 Suppresses Invasion and Migration via E2F3 in Nasopharyngeal Carcinoma.Onco Targets Ther. 2019 Dec 19;12:11271-11280. doi: 10.2147/OTT.S233435. eCollection 2019. Onco Targets Ther. 2019. PMID: 31908492 Free PMC article.
-
Long noncoding RNA PTPRG-AS1 acts as a microRNA-194-3p sponge to regulate radiosensitivity and metastasis of nasopharyngeal carcinoma cells via PRC1.J Cell Physiol. 2019 Aug;234(10):19088-19102. doi: 10.1002/jcp.28547. Epub 2019 Apr 16. J Cell Physiol. 2019. PMID: 30993702
-
Low Expression of BIRC5-206 Promotes Cancer Progression in Nasopharyngeal Carcinoma via Enhancing Expression of Stem Cell Markers.Ann Clin Lab Sci. 2023 May;53(3):380-388. Ann Clin Lab Sci. 2023. PMID: 37437934
-
E2F1-EP300 co-activator complex potentiates immune escape in nasopharyngeal carcinoma through the mediation of MELK.Histol Histopathol. 2024 Apr;39(4):511-523. doi: 10.14670/HH-18-662. Epub 2023 Sep 6. Histol Histopathol. 2024. PMID: 37728155
Cited by
-
Neuron-like macrophage differentiation via the APOE-TREM2 axis contributes to chronic pain in nasopharyngeal carcinoma.Cell Biol Toxicol. 2025 May 20;41(1):86. doi: 10.1007/s10565-025-10035-5. Cell Biol Toxicol. 2025. PMID: 40392335 Free PMC article.
-
A Proteomic Analysis of Nasopharyngeal Carcinoma in a Moroccan Subpopulation.Cancers (Basel). 2024 Sep 26;16(19):3282. doi: 10.3390/cancers16193282. Cancers (Basel). 2024. PMID: 39409902 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64‐80. - PubMed
-
- Lee HM, Okuda KS, Gonzalez FE, Patel V. Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol. 2019;1164:11‐34. - PubMed
-
- Zeng Z, Huang H, Zhang W, et al. Nasopharyngeal carcinoma: advances in genomics and molecular genetics. Sci China Life Sci. 2011;54(10):966‐975. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

