The plant-unique protein DRIF1 coordinates with sorting nexin 1 to regulate membrane protein homeostasis
- PMID: 37647529
- PMCID: PMC10689196
- DOI: 10.1093/plcell/koad227
The plant-unique protein DRIF1 coordinates with sorting nexin 1 to regulate membrane protein homeostasis
Abstract
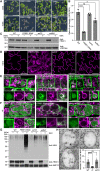
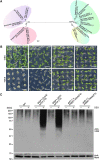
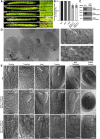
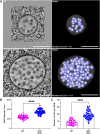
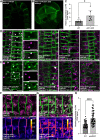
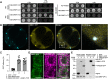
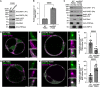
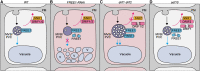
Membrane protein homeostasis is fine-tuned by the cellular pathways for vacuolar degradation and recycling, which ultimately facilitate plant growth and cell-environment interactions. The endosomal sorting complex required for transport (ESCRT) machinery plays important roles in regulating intraluminal vesicle (ILV) formation and membrane protein sorting to vacuoles. We previously showed that the plant-specific ESCRT component FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING1 (FREE1) performs multiple functions in plants, although the underlying mechanisms remain elusive. In this study, we performed a suppressor screen of the FREE1-RNAi mutant and identified and characterized 2 suppressor of free1 (sof) mutants in Arabidopsis (Arabidopsis thaliana). These mutants, sof10 and sof641, result in a premature stop codon or a missense mutation in AT5G10370, respectively. This gene was named DEAH and RING domain-containing protein as FREE1 suppressor 1 (DRIF1). DRIF1 has a homologous gene, DRIF2, in the Arabidopsis genome with 95% identity to DRIF1. The embryos of drif1 drif2 mutants arrested at the globular stage and formed enlarged multivesicular bodies (MVBs) with an increased number of ILVs. DRIF1 is a membrane-associated protein that coordinates with retromer component sorting nexin 1 to regulate PIN-FORMED2 recycling to the plasma membrane. Altogether, our data demonstrate that DRIF1 is a unique retromer interactor that orchestrates FREE1-mediated ILV formation of MVBs and vacuolar sorting of membrane proteins for degradation in plants.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

