Association of GLP-1 Receptor Agonists with Chronic Obstructive Pulmonary Disease Exacerbations among Patients with Type 2 Diabetes
- PMID: 37647574
- PMCID: PMC10867930
- DOI: 10.1164/rccm.202303-0491OC
Association of GLP-1 Receptor Agonists with Chronic Obstructive Pulmonary Disease Exacerbations among Patients with Type 2 Diabetes
Abstract
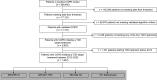
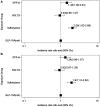
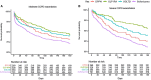
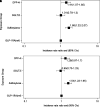
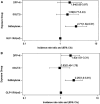
Rationale: Patients with chronic obstructive pulmonary disease (COPD) and type 2 diabetes (T2D) have worse clinical outcomes compared with patients without metabolic dysregulation. GLP-1 (glucagon-like peptide 1) receptor agonists (GLP-1RAs) reduce asthma exacerbation risk and improve FVC in patients with COPD. Objectives: To determine whether GLP-1RA use is associated with reduced COPD exacerbation rates, and severe and moderate exacerbation risk, compared with other T2D therapies. Methods: A retrospective, observational, electronic health records-based study was conducted using an active comparator, new-user design of 1,642 patients with COPD in a U.S. health system from 2012 to 2022. The COPD cohort was identified using a previously validated machine learning algorithm that includes a natural language processing tool. Exposures were defined as prescriptions for GLP-1RAs (reference group), DPP-4 (dipeptidyl peptidase 4) inhibitors (DPP-4is), SGLT2 (sodium-glucose cotransporter 2) inhibitors, or sulfonylureas. Measurements and Main Results: Unadjusted COPD exacerbation counts were lower in GLP-1RA users. Adjusted exacerbation rates were significantly higher in DPP-4i (incidence rate ratio, 1.48 [95% confidence interval, 1.08-2.04]; P = 0.02) and sulfonylurea (incidence rate ratio, 2.09 [95% confidence interval, 1.62-2.69]; P < 0.0001) users compared with GLP-1RA users. GLP-1RA use was also associated with significantly reduced risk of severe exacerbations compared with DPP-4i and sulfonylurea use, and of moderate exacerbations compared with sulfonylurea use. After adjustment for clinical covariates, moderate exacerbation risk was also lower in GLP-1RA users compared with DPP-4i users. No statistically significant difference in exacerbation outcomes was seen between GLP-1RA and SGLT2 inhibitor users. Conclusions: Prospective studies of COPD exacerbations in patients with comorbid T2D are warranted. Additional research may elucidate the mechanisms underlying these observed associations with T2D medications.
Keywords: health services research; obesity; obstructive lung diseases; type 2 diabetes mellitus.
Figures
Comment in
-
Improving Outcomes of Chronic Obstructive Pulmonary Disease through the Treatment of Comorbidities: One Step Beyond.Am J Respir Crit Care Med. 2023 Nov 15;208(10):1017-1019. doi: 10.1164/rccm.202309-1546ED. Am J Respir Crit Care Med. 2023. PMID: 37672750 Free PMC article. No abstract available.
-
A Daunting and Challenging Task to Prove the Effectiveness of Reducing Acute Exacerbation in COPD Patients with Type 2 Diabetes by GLP-1 Receptor Agonists.Am J Respir Crit Care Med. 2023 Dec 15;208(12):1345-1346. doi: 10.1164/rccm.202309-1671LE. Am J Respir Crit Care Med. 2023. PMID: 37855726 Free PMC article. No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease. 2023. https://www.goldcopd.org/wp-content/uploads/2022/12/GOLD-2023-ver-1.1-2D...
-
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res . 2010;11:122. - PubMed
-
- Belligund P, Attaway A, Lopez R, Damania D, Hatipoğlu U, Zein JG. Diabetes associated with higher health care utilization and poor outcomes after COPD-related hospitalizations. Am J Manag Care . 2022;28:e325–e332. - PubMed
-
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab . 2018;27:740–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI155299/AI/NIAID NIH HHS/United States
- R01 HL117945/HL/NHLBI NIH HHS/United States
- U19 AI095219/AI/NIAID NIH HHS/United States
- P30 AR070253/AR/NIAMS NIH HHS/United States
- K23 HL161332/HL/NHLBI NIH HHS/United States
- R01 AI078908/AI/NIAID NIH HHS/United States
- U01 AI1155299/HG/NHGRI NIH HHS/United States
- R01 HL136209/HL/NHLBI NIH HHS/United States
- U01HG008685/HG/NHGRI NIH HHS/United States
- R01 AI136041/AI/NIAID NIH HHS/United States
- K23HL161332/HL/NHLBI NIH HHS/United States
- R37 AI052353/AI/NIAID NIH HHS/United States
- U01 HG008685/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

