Pathogenic Rickettsia, Anaplasma, and Ehrlichia in Rhipicephalus microplus ticks collected from cattle and laboratory hatched tick larvae
- PMID: 37647577
- PMCID: PMC10468208
- DOI: 10.1371/journal.pntd.0011546
Pathogenic Rickettsia, Anaplasma, and Ehrlichia in Rhipicephalus microplus ticks collected from cattle and laboratory hatched tick larvae
Abstract
Background: The order Rickettsiales contains a group of vector-borne gram-negative obligate intracellular bacteria, which often cause human emerging infectious diseases and economic losses for dairy and meat industries. The purpose of this study is to investigate the distribution of the pathogens including Rickettsia spp., Anaplasma spp., and Ehrlichia spp. in the order Rickettsiales in ticks from Yueyang, a prefecture-level city of Hunan Province in Sothern China, and assess the potentiality of transovarial transmission of these rickettsial organisms.
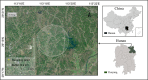
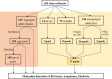
Methods: Ticks were collected from cattle in a farm in Yueyang City and the tick DNA was used as template to amplify the htrA, rrs, gltA, ompA and ompB genes of Rickettsia as well as rrs and groEL genes of Anaplasma and Ehrlichia.
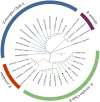
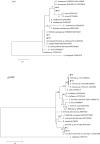
Results: All ticks (465) collected were the cattle tick, Rhipicephalus microplus. PCR showed the minimum infection rate (MIR) was 1.5% (7/465) for Candidatus Rickettsia xinyangensis, 1.9% (9/465) for C. Anaplasma boleense, 1.3% (6/465) for Anaplasma platys, 0.6% (3/465) for A. marginale, and 1.17% (2/465) for each of A. bovis, Ehrlichia minasensis, and a non-classified Ehrlichia sp. A human pathogen, C. Rickettsia xinyangensis and A. platys were detected in 100% (3/3) and 33.3% (2/6) laboratory-hatched larval pools from infected females respectively.
Conclusion: Our study revealed a diversity of pathogenic rickettsial species in R. microplus ticks from Hunan Province suggesting a threat to people and animals in China. This study also provided the first molecular evidence for the potential transovarial transmission of C. Rickettsia xinyangensis and A. platys in R. microplus, indicating that R. microplus may act as the host of these two pathogens.
Copyright: © 2023 Xu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

