A phase 2 trial of CD24Fc for prevention of graft-versus-host disease
- PMID: 37647633
- PMCID: PMC10934299
- DOI: 10.1182/blood.2023020250
A phase 2 trial of CD24Fc for prevention of graft-versus-host disease
Abstract
Patients who undergo human leukocyte antigen-matched unrelated donor (MUD) allogeneic hematopoietic stem cell transplantation (HSCT) with myeloablative conditioning for hematologic malignancies often develop acute graft-versus-host disease (GVHD) despite standard calcineurin inhibitor-based prophylaxis in combination with methotrexate. This trial evaluated a novel human CD24 fusion protein (CD24Fc/MK-7110) that selectively targets and mitigates inflammation due to damage-associated molecular patterns underlying acute GVHD while preserving protective immunity after myeloablative conditioning. This phase 2a, multicenter study evaluated the pharmacokinetics, safety, and efficacy of CD24Fc in combination with tacrolimus and methotrexate in preventing acute GVHD in adults undergoing MUD HSCT for hematologic malignancies. A double-blind, placebo-controlled, dose-escalation phase to identify a recommended dose was followed by an open-label expansion phase with matched controls to further evaluate the efficacy and safety of CD24Fc in preventing acute GVHD. A multidose regimen of CD24Fc produced sustained drug exposure with similar safety outcomes when compared with single-dose regimens. Grade 3 to 4 acute GVHD-free survival at day 180 was 96.2% (95% confidence interval [CI], 75.7-99.4) in the CD24Fc expansion cohort (CD24Fc multidose), compared with 73.6% (95% CI, 63.2-81.4) in matched controls (hazard ratio, 0.1 [95% CI, 0.0-0.6]; log-rank test, P = .03). No participants in the CD24Fc escalation or expansion phases experienced dose-limiting toxicities (DLTs). The multidose regimen of CD24Fc was well tolerated with no DLTs and was associated with high rates of severe acute GVHD-free survival after myeloablative MUD HSCT. This trial was registered at ClinicalTrials.gov as #NCT02663622.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: J. Magenau has received research funding from Merck. S.A. has served as site principal investigator for AltruBio, Inc; CSL Behring, LLC; Equilibrium, Inc; and Incyte Corporation. S.L., B.B., and C.D. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, who may own stock and/or hold stock options in Merck & Co, Inc, Rahway, NJ. M.D. is a former employee of OncoImmune and had stock options from OncoImmune during the study period as an employee. S.D. is an employee of the National Marrow Donor Program, which supplied matched unrelated donor grafts to US transplantation centers participating in this study. Y.L. and P.Z. are founders and former part-time employees of OncoImmune; they held stocks and stock options from OncoImmune during the study period as employees; and they served as coinvestigators for a grant from the National Institutes of Health/National Cancer Institute (R44CA221513). Y.L. received funding from the US Food and Drug Administration (R01FD006089-01). P.Z. received funding from the National Institutes of Health/National Cancer Institute (R44CA246991). The remaining authors declare no competing financial interests.
The current affiliation of P.R. is Baylor College of Medicine, Houston, TX.
Figures


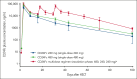

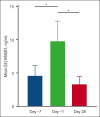
Comment in
-
CD24Fc to DAMPen GVHD.Blood. 2024 Jan 4;143(1):1-2. doi: 10.1182/blood.2023022228. Blood. 2024. PMID: 38175676 No abstract available.
References
-
- Center for International Blood and Marrow Transplant Research The US summary slides - HCT trends and survival data. https://cibmtr.org/CIBMTR/Resources/Summary-Slides-Reports
-
- Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. - PubMed
-
- Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24(36):5695–5702. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

