A Pilot Single-Site Randomized Control Trial: Investigating the Use of Donor Milk in Late Preterm and Term Infants in the Neonatal Intensive Care Unit
- PMID: 37647913
- PMCID: PMC12068505
- DOI: 10.1055/s-0043-1771261
A Pilot Single-Site Randomized Control Trial: Investigating the Use of Donor Milk in Late Preterm and Term Infants in the Neonatal Intensive Care Unit
Abstract
Objective: We aimed to study donor milk (DM) supplementation when mother's own milk (MOM) was unavailable in term and late preterm infants (LPIs) admitted to the neonatal intensive care unit (NICU). We hypothesized that this study would be feasible, defined by the rate of consent, diet adherence, and study completion. We further hypothesized that compared with formula supplementation, DM supplementation, for no longer than 7 days from birth, would be associated with an increase in breastfeeding attempts and the percentage of MOM (MOM%) without adversely affecting growth. Breastfeeding attempts and MOM% were assessed over 48 hours at the end of the intervention, which was defined as NICU discharge or at the end of supplementation, whichever came sooner.
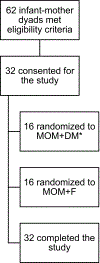
Study design: This was a pilot study (n = 32). Infants with a gestational age > 34 weeks admitted to the NICU were included. Infants were randomized to one of two groups: human milk (MOM + DM) or formula (MOM + F).
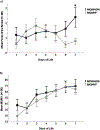
Results: The consent rate was 52%. Adherence to the study diet was 97%, and completion was 100%. When the MOM + DM group was compared with the MOM + F group, there was no difference in breastfeeding attempts (median [interquartile range]: 3.5 [1.5-6] vs. 1.5 [0.5-4] times, p = 0.1) or MOM% (60 vs. 59%, p = 0.9). Weight and length at multiple time points were similar when the groups were compared.
Conclusion: A study randomizing term and LPIs in the NICU to DM or formula when MOM was unavailable is feasible. It remains unclear if DM improves breastfeeding success in this population.
Key points: · A study that randomizes term and late preterm infants in the NICU to DM or formula supplementation when mother's own milk is not available is feasible.. · It remains unclear if DM compared to formula supplementation improves direct breastfeeding.. · In general, growth was similar in infants who received DM or formula as a supplement..
Thieme. All rights reserved.
Conflict of interest statement
K.L.C. has received research support from Fresenius Kabi. K.L.C. has served as an advisor for Fresenius Kabi, Mead Johnson, Baxter, and Prolacta. K.L.C. serves as an institutional principal investigator, with no salary funding, for a consortium database sponsored by Mead Johnson.
Figures
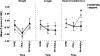
Update of
-
A Pilot Single-Site Randomized Control Trial: Investigating the Use of Donor Milk in the Late Preterm and Term Infant in the Neonatal Intensive Care Unit.Res Sq [Preprint]. 2023 Feb 8:rs.3.rs-2540272. doi: 10.21203/rs.3.rs-2540272/v1. Res Sq. 2023. Update in: Am J Perinatol. 2024 May;41(S 01):e2427-e2435. doi: 10.1055/s-0043-1771261. PMID: 36798190 Free PMC article. Updated. Preprint.
Similar articles
-
A Pilot Single-Site Randomized Control Trial: Investigating the Use of Donor Milk in the Late Preterm and Term Infant in the Neonatal Intensive Care Unit.Res Sq [Preprint]. 2023 Feb 8:rs.3.rs-2540272. doi: 10.21203/rs.3.rs-2540272/v1. Res Sq. 2023. Update in: Am J Perinatol. 2024 May;41(S 01):e2427-e2435. doi: 10.1055/s-0043-1771261. PMID: 36798190 Free PMC article. Updated. Preprint.
-
Association between Early Feeding Patterns and Neonatal Outcomes in Very Preterm Infants: A Retrospective Cohort Study.Neonatology. 2023;120(1):71-80. doi: 10.1159/000527522. Epub 2023 Jan 5. Neonatology. 2023. PMID: 36603553
-
Impact of Donor Milk on Short- and Long-Term Growth of Very Low Birth Weight Infants.Nutrients. 2019 Jan 22;11(2):241. doi: 10.3390/nu11020241. Nutrients. 2019. PMID: 30678256 Free PMC article.
-
Racial and socioeconomic disparities in breast milk feedings in US neonatal intensive care units.Pediatr Res. 2021 Jan;89(2):344-352. doi: 10.1038/s41390-020-01263-y. Epub 2020 Nov 13. Pediatr Res. 2021. PMID: 33188286 Free PMC article. Review.
-
Benefits of donor milk in the feeding of preterm infants.Early Hum Dev. 2013 Oct;89 Suppl 2:S3-6. doi: 10.1016/j.earlhumdev.2013.07.008. Epub 2013 Aug 6. Early Hum Dev. 2013. PMID: 23932110 Review.
Cited by
-
Donor human milk versus infant formula for low-risk infants: a systematic review.Pediatr Res. 2025 Jan;97(1):81-91. doi: 10.1038/s41390-024-03309-x. Epub 2024 Jun 6. Pediatr Res. 2025. PMID: 38844541 Free PMC article.
-
Supplemental Donor Milk vs Infant Formula in Moderate to Late Preterm Infants: A Randomized Clinical Trial.JAMA Pediatr. 2025 Aug 4:e252365. doi: 10.1001/jamapediatrics.2025.2365. Online ahead of print. JAMA Pediatr. 2025. PMID: 40758360 Free PMC article.
-
Formula protein versus human milk protein and the effects on growth in preterm born infants.Curr Opin Clin Nutr Metab Care. 2025 Jan 1;28(1):33-38. doi: 10.1097/MCO.0000000000001084. Epub 2024 Nov 11. Curr Opin Clin Nutr Metab Care. 2025. PMID: 39659212 Free PMC article. Review.
References
-
- Kachoria R, Oza-Frank R. Trends in breastfeeding initiation in the NICU by gestational age in Ohio, 2006–2012. Birth. 2015;42(1):56–61. - PubMed
-
- De Halleux V, Pieltain C, Senterre T, Rigo J. Use of donor milk in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2017;22(1):23–29. - PubMed
-
- Arslanoglu S, Moro GE, Bellù R, Turoli D, De Nisi G, Tonetto P, et al. Presence of human milk bank is associated with elevated rate of exclusive breastfeeding in VLBW infants. J Perinat Med 2012;15:1–3. - PubMed
-
- McCune S, Perrin MT. Donor human milk use in populations other than the preterm infant: A systematic scoping review. Breastfeed Med. 2021. Jan;16(1):8–20. - PubMed

