Aberrant Effective Connectivity During Eye Gaze Processing Is Linked to Social Functioning and Symptoms in Schizophrenia
- PMID: 37648206
- PMCID: PMC10840731
- DOI: 10.1016/j.bpsc.2023.08.004
Aberrant Effective Connectivity During Eye Gaze Processing Is Linked to Social Functioning and Symptoms in Schizophrenia
Abstract
Background: Patients with schizophrenia show abnormal gaze processing, which is associated with social dysfunction. These abnormalities are related to aberrant connectivity among brain regions that are associated with visual processing, social cognition, and cognitive control. In this study, we investigated 1) how effective connectivity during gaze processing is disrupted in schizophrenia and 2) how this may contribute to social dysfunction and clinical symptoms.
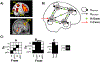
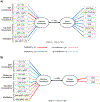
Methods: Thirty-nine patients with schizophrenia/schizoaffective disorder (SZ) and 33 healthy control participants completed an eye gaze processing task during functional magnetic resonance imaging. Participants viewed faces with different gaze angles and performed explicit and implicit gaze processing. Four brain regions-the secondary visual cortex, posterior superior temporal sulcus, inferior parietal lobule, and posterior medial frontal cortex-were identified as nodes for dynamic causal modeling analysis.
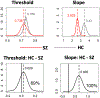
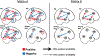
Results: Both the SZ and healthy control groups showed similar model structures for general gaze processing. Explicit gaze discrimination led to changes in effective connectivity, including stronger excitatory, bottom-up connections from the secondary visual cortex to the posterior superior temporal sulcus and inferior parietal lobule and inhibitory, top-down connections from the posterior medial frontal cortex to the secondary visual cortex. Group differences in top-down modulation from the posterior medial frontal cortex to the posterior superior temporal sulcus and inferior parietal lobule were noted, such that these inhibitory connections were attenuated in the healthy control group but further strengthened in the SZ group. Connectivity was associated with social dysfunction and symptom severity.
Conclusions: The SZ group showed notably stronger top-down inhibition during explicit gaze discrimination, which was associated with more social dysfunction but less severe symptoms among patients. These findings help pinpoint neural mechanisms of aberrant gaze processing and may serve as future targets for interventions that combine neuromodulation with social cognitive training.
Keywords: DCM; Face processing; Gaze; Schizophrenia; Social cognition; fMRI.
Copyright © 2023 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest to report.
Figures


Comment in
-
Linking Neural Connectivity Patterns With Real-World Social Performance: The Relevance of Ecological Measures.Biol Psychiatry Cogn Neurosci Neuroimaging. 2023 Dec;8(12):1171-1172. doi: 10.1016/j.bpsc.2023.10.003. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023. PMID: 38061814 No abstract available.
Similar articles
-
Dynamic causal modeling of eye gaze processing in schizophrenia.Schizophr Res. 2021 Mar;229:112-121. doi: 10.1016/j.schres.2020.11.012. Epub 2020 Nov 20. Schizophr Res. 2021. PMID: 33229223 Free PMC article.
-
Neural Oscillatory Abnormalities During Gaze Processing in Schizophrenia: Evidence of Reduced Theta Phase Consistency and Inter-areal Theta-Gamma Coupling.Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 Mar;6(3):370-379. doi: 10.1016/j.bpsc.2020.08.013. Epub 2020 Sep 1. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021. PMID: 33160880 Free PMC article.
-
Neural circuit disruptions of eye gaze processing in autism spectrum disorder and schizophrenia: An activation likelihood estimation meta-analysis.Schizophr Res. 2024 Feb;264:298-313. doi: 10.1016/j.schres.2023.12.003. Epub 2024 Jan 11. Schizophr Res. 2024. PMID: 38215566 Free PMC article.
-
The influence of top-down modulation on the processing of direct gaze.Wiley Interdiscip Rev Cogn Sci. 2019 Sep;10(5):e1500. doi: 10.1002/wcs.1500. Epub 2019 Mar 12. Wiley Interdiscip Rev Cogn Sci. 2019. PMID: 30864304 Review.
-
Human face and gaze perception is highly context specific and involves bottom-up and top-down neural processing.Neurosci Biobehav Rev. 2022 Jan;132:304-323. doi: 10.1016/j.neubiorev.2021.11.042. Epub 2021 Nov 30. Neurosci Biobehav Rev. 2022. PMID: 34861296 Review.
Cited by
-
Social stress in schizophrenia: Unique contributions to social cognition and social functioning.Schizophr Res. 2025 Feb;276:167-174. doi: 10.1016/j.schres.2025.01.015. Epub 2025 Jan 31. Schizophr Res. 2025. PMID: 39892250
-
Altered hippocampal effective connectivity predicts BMI and food approach behavior in children with obesity.Int J Clin Health Psychol. 2025 Jan-Mar;25(1):100541. doi: 10.1016/j.ijchp.2024.100541. Epub 2025 Jan 10. Int J Clin Health Psychol. 2025. PMID: 39877891 Free PMC article.
-
Messiah or pariah? Psychosis, science, and finding meaning in lived experience.Schizophrenia (Heidelb). 2024 Aug 5;10(1):67. doi: 10.1038/s41537-024-00486-w. Schizophrenia (Heidelb). 2024. PMID: 39103367 Free PMC article.
-
Constructs across a hierarchical, dimensional model of psychopathology show differential associations with social and general cognitive ability.PLoS One. 2025 Jan 22;20(1):e0317377. doi: 10.1371/journal.pone.0317377. eCollection 2025. PLoS One. 2025. PMID: 39841648 Free PMC article.
References
-
- Green MF, Horan WP, Lee J (2015): Social cognition in schizophrenia [no. 10]. Nat Rev Neurosci 16: 620–631. - PubMed
-
- Harvey PD, Patterson TL, Potter LS, Zhong K, Brecher M (2006): Improvement in Social Competence With Short-Term Atypical Antipsychotic Treatment: A Randomized, Double-Blind Comparison of Quetiapine Versus Risperidone for Social Competence, Social Cognition, and Neuropsychological Functioning. Am J Psychiatry 163: 1918–1925. - PubMed
-
- Fett A-KJ, Viechtbauer W, Dominguez M-G, Penn DL, van Os J, Krabbendam L (2011): The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci Biobehav Rev 35: 573–588. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

