Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3
- PMID: 37648261
- PMCID: PMC10471850
- DOI: 10.1136/jitc-2023-007118
Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3
Abstract
Background: Brexucabtagene autoleucel (brexu-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the USA for adults with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) and in the European Union for patients ≥26 years with R/R B-ALL. After 2 years of follow-up in ZUMA-3, the overall complete remission (CR) rate (CR+CR with incomplete hematological recovery (CRi)) was 73%, and the median overall survival (OS) was 25.4 months in 78 Phase 1 and 2 patients with R/R B-ALL who received the pivotal dose of brexu-cel. Outcomes by prior therapies and subsequent allogeneic stem cell transplantation (alloSCT) are reported.
Methods: Eligible adults had R/R B-ALL and received one infusion of brexu-cel (1×10⁶ CAR T cells/kg) following conditioning chemotherapy. The primary endpoint was the CR/CRi rate per central review. Post hoc subgroup analyses were exploratory with descriptive statistics provided.
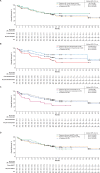
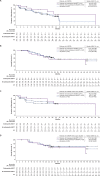
Results: Phase 1 and 2 patients (N=78) were included with median follow-up of 29.7 months (range, 20.7-58.3). High CR/CRi rates were observed across all prior therapy subgroups examined: 1 prior line of therapy (87%, n=15) and ≥2 prior lines (70%, n=63); prior blinatumomab (63%, n=38) and no prior blinatumomab (83%, n=40); prior inotuzumab (59%, n=17) and no prior inotuzumab (77%, n=61); and prior alloSCT (76%, n=29) and no prior alloSCT (71%, n=49). The frequency of Grade ≥3 cytokine release syndrome, neurological events, and treatment-related Grade 5 adverse events were largely similar among prior therapy subgroups.Median duration of remission (DOR) in responders with (n=14) and without (n=43) subsequent alloSCT was 44.2 (95% CI, 8.1 to not estimable (NE)) and 18.6 months (95% CI, 9.4 to NE); median OS was 47.0 months (95% CI, 10.2 to NE) and not reached (95% CI, 23.2 to NE), respectively. Median DOR and OS were not reached in responders without prior or subsequent alloSCT (n=22).
Conclusions: In ZUMA-3, adults with R/R B-ALL benefited from brexu-cel, regardless of prior therapies and subsequent alloSCT status, though survival appeared better in patients without certain prior therapies and in earlier lines of therapy. Additional studies are needed to determine the impact prior therapies and subsequent alloSCT have on outcomes of patients who receive brexu-cel.
Keywords: Cell Engineering; Cytotoxicity, Immunologic; Hematologic Neoplasms; Immunity, Cellular; Immunotherapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BS reports honoraria from Acrotech Biopharma, BeiGene, Gilead Sciences, Janssen, Pharmacyclics, and Spectrum; consulting/advisory role for Adaptive Biotechnologies; Amgen; Celgene/BMS; Kite, a Gilead Company; Novartis; Pfizer; and Precision BioSciences; research funding from Gilead Sciences, Incyte, Jazz Pharmaceuticals, and Kite; and travel support from Celgene, Janssen, Kite, Novartis, Pfizer, Seattle Genetics, and Stemline Therapeutics. RC reports spouse employment with Seagen; stock or other ownership in Seagen; honoraria from Amgen, Autolus (board member), PeproMene Bio (board member), Pfizer, and Kite; consultancy/advisory role for Amgen and Jazz; and research funding from Pfizer, Merck, Amgen, Servier, Kite, and Vanda. JHP reports consulting/advisory role for AstraZeneca, Kite, and Novartis and research funding from Amgen, Genentech, and Juno. RH reports honoraria from ADC therapeutics, Bristol Myers Squibb, Celgene, Gilead Sciences, Janssen, Kite, MSD, Novartis, and Roche; and consulting/advisory role for Kite/Gilead. OOO reports consulting/advisory role for Pfizer, Kite, Gilead, AbbVie, Janssen, TG Therapeutics, ADC, Novartis, Epizyme, Curio Science, Nektar, and Syncopation; research funding from Kite, Pfizer, Daiichi Sankyo, and Allogene; and honoraria from Pfizer and Gilead. ACL reports consulting/advisory role for AbbVie, Bristol Myers Squibb, and Pfizer; research funding from Amgen, Amphivena, Astellas, Autolus, Jazz, Kadmon, Kite, and Pharmacyclics. NB reports honoraria from and consulting/advisory role for Gilead Sciences. TL reports consultancy/advisory role for Amgen and Servier. MRBi reports honoraria from Incyte, Bristol Myers Squibb, Sanofi, and ADC Therapeutics; consulting/advisory role for Novartis, Kite/Gilead, Bristol Myers Squibb, Sana Bio, and CRISPR Therapeutics; and speakers’ bureau participation for Incyte, Bristol Myers Squibb, Sanofi, and ADC Therapeutics. MST reports consulting/advisory role for Amgen, Celgene, Kite, Regeneron, and Roche and research funding from Amgen, Kite, MacroGenics, Regeneron, and Roche. DT reports consulting/advisory role for Bristol Myers Squibb, EUSA, Partner, and Takeda; and research funding from Bristol Myers Squibb. KMO reports consulting/advisory role for Beam Therapeutics. MLA reports consulting/advisory role for Kite and Syndax Pharmaceuticals, Inc. and research funding from Kite (institutional PI). YL reports consulting/advisory role for Kite/Gilead, Celgene/BMS, Juno/BMS, bluebird bio, Janssen, Legend Biotech, Gamida Cell, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer and research funding from Kite/Gilead, Celgene/BMS, bluebird bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. MRBa reports research funding from AbbVie, Ascentage, Kite, Kura, and Takeda. GJS reports honoraria and research funding from and speakers’ bureau participation for Kite. MS reports consulting/advisory role for Amgen, Celgene/BMS, Gilead Sciences, Janssen, and Novartis; speakers’ bureau participation for Celgene/BMS, Gilead Sciences, Janssen, and Novartis; research funding from Amgen, Gilead Sciences, Miltenyi Biotec, MorphoSys, Roche, and Seattle Genetics; and travel support from Takeda. MA reports stock or other ownership in CytoDyn; consulting/advisory role for Celgene; and speakers’ bureau participation for Celgene, Bristol Myers Squibb, AbbVie, and Kite. MCM reports consulting/advisory role for Gilead Sciences and Janssen-Cilag and speakers’ bureau participation for Janssen-Cilag and Medscape. WW reports consulting/advisory role for Genzyme and Sanofi and research funding from AbbVie, Acerta, Cyclacel, Genentech, Gilead Sciences, GSK, Janssen, Juno, Karyopharm, Loxo Oncology, miRagen, Novartis, Oncternal, Pharmacyclics, Sunesis, and Xencor. DJD reports consulting or advisory role for Agios, Amgen, Autolus, Blueprint Medicines, Forty Seven, Gilead Sciences, Incyte, Jazz, Novartis, Pfizer, Shire, and Takeda and research funding from AbbVie, Glycomimetics, Novartis, and Blueprint Medicines. PJS reports honoraria from and consulting or advisory role for MorphoSys and CRISPR Therapeutics and research funding from Amgen, Gamida Cell, Pfizer, Karyopharm, Gilead Sciences, Incyte, Seagen, and Cellectar. DJ reports research funding from Jazz Pharmaceuticals and Pfizer. DM has nothing to report. SA reports employment with and stock or other ownership in Kite. LZ reports employment with Kite and ownership of AbbVie RSU or stock in the past 2 years. reports employment with Kite; stock or other ownership in Gilead Sciences; and travel support from Kite and Gilead Sciences. RDK reports employment, stock, or other ownership in Kite. AG reports consulting/advisory role for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc.; research funding from Amgen, Genentech, and Kite; and honoraria from Kite.
Figures


References
-
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. . Inotuzumab Ozogamicin versus standard of care in Relapsed or refractory acute Lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019;125:2474–87. 10.1002/cncr.32116 - DOI - PMC - PubMed
-
- Kite Pharma, Inc . TECARTUS® (brexucabtagene autoleucel) [prescribing information]. Santa Monica, CA, 2021.
