Recruiting historically under-represented individuals into Project ECHO Diabetes: using barrier analysis to understand disparities in clinical research in the USA
- PMID: 37648378
- PMCID: PMC10471869
- DOI: 10.1136/bmjopen-2023-072546
Recruiting historically under-represented individuals into Project ECHO Diabetes: using barrier analysis to understand disparities in clinical research in the USA
Abstract
Objectives: Individuals under-recruited in diabetes research studies include those not seen at endocrinology centres and those from rural, low socioeconomic and/or under-represented racial/ethnic groups. The purpose of this descriptive analysis is to detail recruitment and retention efforts of Project ECHO Diabetes clinical sites affiliated with Stanford University and University of Florida.
Design: Prospective collection of participant engagement and qualitative analysis of barriers and facilitators of research engagement within Project ECHO Diabetes, a virtual tele-education programme for healthcare providers in the management of individuals with insulin-requiring diabetes.
Setting: Data were collected at the patient level, provider level and clinic level between 1 May 2021 and 31 July 2022.
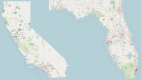
Participants: Participants and study personnel were recruited from 33 Project ECHO Diabetes sites in California and Florida.
Outcomes: We report study completion rates for participants recruited into 33 Project ECHO Diabetes sites. Using barrier analysis, a methodology designed for the real-time assessment of interventions and system processes to identify barriers and facilitators, study personnel identified significant barriers to recruitment and retention and mapped them to actionable solutions.
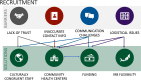
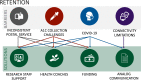
Results: In total, 872 participants (California n=495, Florida n=377) were recruited with differing recruitment rates by site (California=52.7%, Florida=21.5%). Barrier analysis identified lack of trust, unreliable contact information, communication issues and institutional review board (IRB) requirements as key recruitment barriers. Culturally congruent staff, community health centre (CHC) support, adequate funding and consent process flexibility were solutions to address recruitment challenges. Barriers to retention were inconsistent postal access, haemoglobin A1c kit collection challenges, COVID-19 pandemic and broadband/connectivity issues. Additional funding supporting research staff and analogue communication methods were identified as solutions address barriers to retention.
Conclusions: Funded partnerships with CHCs, trusted by their local communities, were key in our recruitment and retention strategies. IRB consent process flexibility reduced barriers to recruitment. Recruiting historically under-represented populations is feasible with funding aimed to address structural barriers to research participation.
Keywords: Clinical Trial; DIABETES & ENDOCRINOLOGY; Health Equity; Patient Participation; STATISTICS & RESEARCH METHODS.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RL has consulted for Abbott Diabetes Care, Biolinq, Capillary Biomedical, Deep Valley Labs, Morgan Stanley, Glooko, and Tidepool. DMM has received research support from the National Institutes of Health, JDRF, NSF and the Helmsley Charitable Trust; and his institution has received research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem and Roche. He has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, and Insulet, and is supported by grant number P30DK116074. MJH has reserved research support from the NIH, JDRF and the Helmsley Charitable Trust and has been a consultant for Mannkind and Sanofi. Theremaining authors have no potential conflicts of interest relevant to this article.
Figures
References
-
- Applying concepts of Generalizability theory on clinical trial data to investigate sources of variation and their impact on reliability - Vangeneugden - 2005 - Biometrics. Wiley Online Library [Internet]. Available: https://onlinelibrary.wiley.com/doi/full/10.1111/j.0006-341X.2005.031040.x [Accessed 11 Aug 2022]. - PubMed
-
- Institute of Medicine (US) . Committee on understanding and eliminating racial and ethnic disparities in health care. In: Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. Washington (DC): National Academies Press (US), 2003. Available: http://www.ncbi.nlm.nih.gov/books/NBK220358/ [accessed 19 May 2020]. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
