Prevention of maternal and neonatal death/infections with a single oral dose of azithromycin in women in labour in low-income and middle-income countries (A-PLUS): a study protocol for a multinational, randomised placebo-controlled clinical trial
- PMID: 37648383
- PMCID: PMC10471878
- DOI: 10.1136/bmjopen-2022-068487
Prevention of maternal and neonatal death/infections with a single oral dose of azithromycin in women in labour in low-income and middle-income countries (A-PLUS): a study protocol for a multinational, randomised placebo-controlled clinical trial
Abstract
Introduction: Maternal and neonatal infections are among the most frequent causes of maternal and neonatal mortality, and current antibiotic strategies have been ineffective in preventing many of these deaths. A randomised clinical trial conducted in a single site in The Gambia showed that treatment with an oral dose of 2 g azithromycin versus placebo for all women in labour reduced certain maternal and neonatal infections. However, it is unknown if this therapy reduces maternal and neonatal sepsis and mortality. In a large, multinational randomised trial, we will evaluate the impact of azithromycin given in labour to improve maternal and newborn outcomes.
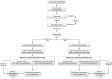
Methods and analysis: This randomised, placebo-controlled, multicentre clinical trial includes two primary hypotheses, one maternal and one neonatal. The maternal hypothesis is to test whether a single, prophylactic intrapartum oral dose of 2 g azithromycin given to women in labour will reduce maternal death or sepsis. The neonatal hypothesis will test whether this intervention will reduce intrapartum/neonatal death or sepsis. The intervention is a single, prophylactic intrapartum oral dose of 2 g azithromycin, compared with a single intrapartum oral dose of an identical appearing placebo. A total of 34 000 labouring women from 8 research sites in sub-Saharan Africa, South Asia and Latin America will be randomised with a one-to-one ratio to intervention/placebo. In addition, we will assess antimicrobial resistance in a sample of women and their newborns.
Ethics and dissemination: The study protocol has been reviewed and ethics approval obtained from all the relevant ethical review boards at each research site. The results will be disseminated via peer-reviewed journals and national and international scientific forums.
Trial registration number: NCT03871491 (https://clinicaltrials.gov/ct2/show/NCT03871491?term=NCT03871491&draw=2&rank=1).
Keywords: Clinical trials; Maternal medicine; NEONATOLOGY; OBSTETRICS; PUBLIC HEALTH.
Link or Companion Paper: bmjopen-2022-067581"Copyright may not be established in the United States for works of government employees (17 U.S.C. § 105). The NIH Public Access Policy requires NIH authors to submit final, peer-reviewed journal manuscripts to the digital archive PubMed Central upon acceptance for publication but no later than 12 months after publication.''.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization . WHO recommendations for the prevention and treatment of maternal peripartum infections; 2015. Available: https://www.who.int/publications/i/item/9789241549363 [Accessed 22 Aug 2018]. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 HD076461/HD/NICHD NIH HHS/United States
- INV-008973/GATES/Gates Foundation/United States
- U10 HD078438/HD/NICHD NIH HHS/United States
- UG1 HD078439/HD/NICHD NIH HHS/United States
- U10 HD076461/HD/NICHD NIH HHS/United States
- U10 HD076465/HD/NICHD NIH HHS/United States
- UG1 HD076465/HD/NICHD NIH HHS/United States
- U10 HD076457/HD/NICHD NIH HHS/United States
- UG1 HD096730/HD/NICHD NIH HHS/United States
- U01 HD040636/HD/NICHD NIH HHS/United States
- U10 HD078437/HD/NICHD NIH HHS/United States
- U10 HD076474/HD/NICHD NIH HHS/United States
- U10 HD078439/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical