Rapid, Activity-Dependent Intrinsic Plasticity in the Developing Zebra Finch Auditory Cortex
- PMID: 37648449
- PMCID: PMC10573762
- DOI: 10.1523/JNEUROSCI.0354-23.2023
Rapid, Activity-Dependent Intrinsic Plasticity in the Developing Zebra Finch Auditory Cortex
Abstract
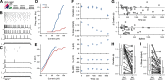
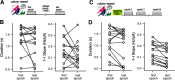
The acoustic environment an animal experiences early in life shapes the structure and function of its auditory system. This process of experience-dependent development is thought to be primarily orchestrated by potentiation and depression of synapses, but plasticity of intrinsic voltage dynamics may also contribute. Here, we show that in juvenile male and female zebra finches, neurons in a cortical-level auditory area, the caudal mesopallium (CM), can rapidly change their firing dynamics. This plasticity was only observed in birds that were reared in a complex acoustic and social environment, which also caused increased expression of the low-threshold potassium channel Kv1.1 in the plasma membrane and endoplasmic reticulum (ER). Intrinsic plasticity depended on activity, was reversed by blocking low-threshold potassium currents, and was prevented by blocking intracellular calcium signaling. Taken together, these results suggest that Kv1.1 is rapidly mobilized to the plasma membrane by activity-dependent elevation of intracellular calcium. This produces a shift in the excitability and temporal integration of CM neurons that may be permissive for auditory learning in complex acoustic environments during a crucial period for the development of vocal perception and production.SIGNIFICANCE STATEMENT Neurons can change not only the strength of their connections to other neurons, but also how they integrate synaptic currents to produce patterns of action potentials. In contrast to synaptic plasticity, the mechanisms and functional roles of intrinisic plasticity remain poorly understood. We found that neurons in the zebra finch auditory cortex can rapidly shift their spiking dynamics within a few minutes in response to intracellular stimulation. This plasticity involves increased conductance of a low-threshold potassium current associated with the Kv1.1 channel, but it only occurs in birds reared in a rich acoustic environment. Thus, auditory experience regulates a mechanism of neural plasticity that allows neurons to rapidly adapt their firing dynamics to stimulation.
Keywords: Kv1.1; activity-dependent plasticity; auditory learning; intrinsic dynamics; zebra finch.
Copyright © 2023 the authors.
Figures






Similar articles
-
Experience- and Sex-Dependent Intrinsic Plasticity in the Zebra Finch Auditory Cortex during Song Memorization.J Neurosci. 2020 Mar 4;40(10):2047-2055. doi: 10.1523/JNEUROSCI.2137-19.2019. Epub 2020 Jan 14. J Neurosci. 2020. PMID: 31937558 Free PMC article.
-
Experience-Dependent Intrinsic Plasticity During Auditory Learning.J Neurosci. 2019 Feb 13;39(7):1206-1221. doi: 10.1523/JNEUROSCI.1036-18.2018. Epub 2018 Dec 12. J Neurosci. 2019. PMID: 30541908 Free PMC article.
-
A Complex Acoustical Environment During Development Enhances Auditory Perception and Coding Efficiency in the Zebra Finch.J Neurosci. 2025 Feb 12;45(7):e1269242024. doi: 10.1523/JNEUROSCI.1269-24.2024. J Neurosci. 2025. PMID: 39730206 Free PMC article.
-
Intrinsic plasticity and birdsong learning.Neurobiol Learn Mem. 2021 Apr;180:107407. doi: 10.1016/j.nlm.2021.107407. Epub 2021 Feb 22. Neurobiol Learn Mem. 2021. PMID: 33631346 Free PMC article. Review.
-
Neuronal mechanisms regulating the critical period of sensory experience-dependent song learning.Neurosci Res. 2019 Mar;140:53-58. doi: 10.1016/j.neures.2018.11.002. Epub 2018 Nov 3. Neurosci Res. 2019. PMID: 30399390 Review.
Cited by
-
A complex acoustical environment during development enhances auditory perception and coding efficiency in the zebra finch.bioRxiv [Preprint]. 2024 Oct 28:2024.06.25.600670. doi: 10.1101/2024.06.25.600670. bioRxiv. 2024. Update in: J Neurosci. 2025 Feb 12;45(7):e1269242024. doi: 10.1523/JNEUROSCI.1269-24.2024. PMID: 38979160 Free PMC article. Updated. Preprint.
-
A complex acoustical environment is necessary for maintenance and development in the zebra finch auditory pallium.bioRxiv [Preprint]. 2025 May 23:2025.05.22.655494. doi: 10.1101/2025.05.22.655494. bioRxiv. 2025. PMID: 40475434 Free PMC article. Preprint.
References
-
- Bishop HI, Guan D, Bocksteins E, Parajuli LK, Murray KD, Cobb MM, Misonou H, Zito K, Foehring RC, Trimmer JS (2015) Distinct cell- and layer-specific expression patterns and independent regulation of Kv2 channel subtypes in cortical pyramidal neurons. J Neurosci 35:14922–14942. 10.1523/JNEUROSCI.1897-15.2015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
