De Novo Mutation Rates in Sticklebacks
- PMID: 37648662
- PMCID: PMC10503787
- DOI: 10.1093/molbev/msad192
De Novo Mutation Rates in Sticklebacks
Abstract
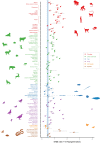
Mutation rate is a fundamental parameter in population genetics. Apart from being an important scaling parameter for demographic and phylogenetic inference, it allows one to understand at what rate new genetic diversity is generated and what the expected level of genetic diversity is in a population at equilibrium. However, except for well-established model organisms, accurate estimates of de novo mutation rates are available for a very limited number of organisms from the wild. We estimated mutation rates (µ) in two marine populations of the nine-spined stickleback (Pungitius pungitius) with the aid of several 2- and 3-generational family pedigrees, deep (>50×) whole-genome resequences and a high-quality reference genome. After stringent filtering, we discovered 308 germline mutations in 106 offspring translating to µ = 4.83 × 10-9 and µ = 4.29 × 10-9 per base per generation in the two populations, respectively. Up to 20% of the mutations were shared by full-sibs showing that the level of parental mosaicism was relatively high. Since the estimated µ was 3.1 times smaller than the commonly used substitution rate, recalibration with µ led to substantial increase in estimated divergence times between different stickleback species. Our estimates of the de novo mutation rate should provide a useful resource for research focused on fish population genetics and that of sticklebacks in particular.
Keywords: divergence time; genetic diversity; germline mutation rate; mutation rate; ninespine stickleback.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures
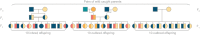


References
-
- Barrett RD, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol Evol. 23:38–44. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

