An interpretable multiparametric radiomics model of basal ganglia to predict dementia conversion in Parkinson's disease
- PMID: 37648733
- PMCID: PMC10468504
- DOI: 10.1038/s41531-023-00566-1
An interpretable multiparametric radiomics model of basal ganglia to predict dementia conversion in Parkinson's disease
Abstract
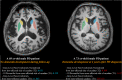
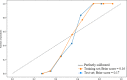
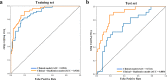
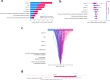
Cognitive impairment in Parkinson's disease (PD) severely affects patients' prognosis, and early detection of patients at high risk of dementia conversion is important for establishing treatment strategies. We aimed to investigate whether multiparametric MRI radiomics from basal ganglia can improve the prediction of dementia development in PD when integrated with clinical profiles. In this retrospective study, 262 patients with newly diagnosed PD (June 2008-July 2017, follow-up >5 years) were included. MRI radiomic features (n = 1284) were extracted from bilateral caudate and putamen. Two models were developed to predict dementia development: (1) a clinical model-age, disease duration, and cognitive composite scores, and (2) a combined clinical and radiomics model. The area under the receiver operating characteristic curve (AUC) were calculated for each model. The models' interpretabilities were studied. Among total 262 PD patients (mean age, 68 years ± 8 [standard deviation]; 134 men), 51 (30.4%), and 24 (25.5%) patients developed dementia within 5 years of PD diagnosis in the training (n = 168) and test sets (n = 94), respectively. The combined model achieved superior predictive performance compared to the clinical model in training (AUCs 0.928 vs. 0.894, P = 0.284) and test set (AUCs 0.889 vs. 0.722, P = 0.016). The cognitive composite scores of the frontal/executive function domain contributed most to predicting dementia. Radiomics derived from the caudate were also highly associated with cognitive decline. Multiparametric MRI radiomics may have an incremental prognostic value when integrated with clinical profiles to predict future cognitive decline in PD.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hely MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov. Disord. 2008;23:837–844. - PubMed
-
- Williams-Gray CH, et al. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. - PubMed
-
- Stoessl AJ, Martin WRW, McKeown MJ, Sossi V. Advances in imaging in Parkinson’s disease. Lancet Neurol. 2011;10:987–1001. - PubMed
-
- McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J. Int. Neuropsychol. Soc. 2010;16:268–277. - PubMed
-
- Chung SJ, et al. Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat. Disord. 2018;51:43–48. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

