A microscale soft ionic power source modulates neuronal network activity
- PMID: 37648756
- PMCID: PMC10468398
- DOI: 10.1038/s41586-023-06295-y
A microscale soft ionic power source modulates neuronal network activity
Abstract
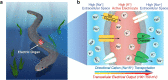
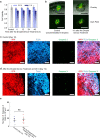
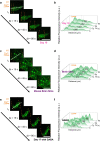
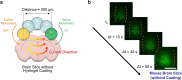
Bio-integrated devices need power sources to operate1,2. Despite widely used technologies that can provide power to large-scale targets, such as wired energy supplies from batteries or wireless energy transduction3, a need to efficiently stimulate cells and tissues on the microscale is still pressing. The ideal miniaturized power source should be biocompatible, mechanically flexible and able to generate an ionic current for biological stimulation, instead of using electron flow as in conventional electronic devices4-6. One approach is to use soft power sources inspired by the electrical eel7,8; however, power sources that combine the required capabilities have not yet been produced, because it is challenging to obtain miniaturized units that both conserve contained energy before usage and are easily triggered to produce an energy output. Here we develop a miniaturized soft power source by depositing lipid-supported networks of nanolitre hydrogel droplets that use internal ion gradients to generate energy. Compared to the original eel-inspired design7, our approach can shrink the volume of a power unit by more than 105-fold and it can store energy for longer than 24 h, enabling operation on-demand with a 680-fold greater power density of about 1,300 W m-3. Our droplet device can serve as a biocompatible and biological ionic current source to modulate neuronal network activity in three-dimensional neural microtissues and in ex vivo mouse brain slices. Ultimately, our soft microscale ionotronic device might be integrated into living organisms.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Similar articles
-
An electric-eel-inspired soft power source from stacked hydrogels.Nature. 2017 Dec 13;552(7684):214-218. doi: 10.1038/nature24670. Nature. 2017. PMID: 29239354 Free PMC article.
-
Powering Electronic Devices from Salt Gradients in AA-Battery-Sized Stacks of Hydrogel-Infused Paper.Adv Mater. 2021 Aug;33(31):e2101757. doi: 10.1002/adma.202101757. Epub 2021 Jun 24. Adv Mater. 2021. PMID: 34165826 Free PMC article.
-
Accordion-Structured Hydrogel Battery Capable of Maintaining Ion Gradients for Extended Periods.ACS Appl Mater Interfaces. 2024 Oct 30;16(43):58617-58627. doi: 10.1021/acsami.4c12092. Epub 2024 Oct 18. ACS Appl Mater Interfaces. 2024. PMID: 39423029
-
Materials Strategies and Device Architectures of Emerging Power Supply Devices for Implantable Bioelectronics.Small. 2020 Apr;16(15):e1902827. doi: 10.1002/smll.201902827. Epub 2019 Sep 12. Small. 2020. PMID: 31513333 Review.
-
Bioinspired Ionic Sensory Systems: The Successor of Electronics.Adv Mater. 2020 Aug;32(31):e2000218. doi: 10.1002/adma.202000218. Epub 2020 Jun 5. Adv Mater. 2020. PMID: 32500602 Review.
Cited by
-
A functional cardiac patch promotes cardiac repair by modulating the CCR2- cardiac-resident macrophage niche and their cell crosstalk.Cell Rep Med. 2025 Feb 18;6(2):101932. doi: 10.1016/j.xcrm.2025.101932. Epub 2025 Jan 28. Cell Rep Med. 2025. PMID: 39879993 Free PMC article.
-
Ion transport and ultra-efficient osmotic power generation in boron nitride nanotube porins.Sci Adv. 2024 Sep 6;10(36):eado8081. doi: 10.1126/sciadv.ado8081. Epub 2024 Sep 6. Sci Adv. 2024. PMID: 39241077 Free PMC article.
-
Recent Progress in Artificial Neurons for Neuromodulation.J Funct Biomater. 2024 Jul 30;15(8):214. doi: 10.3390/jfb15080214. J Funct Biomater. 2024. PMID: 39194652 Free PMC article. Review.
-
Programmed Fabrication of Vesicle-Based Prototissue Fibers with Modular Functionalities.Adv Sci (Weinh). 2025 Apr;12(16):e2409066. doi: 10.1002/advs.202409066. Epub 2025 Feb 10. Adv Sci (Weinh). 2025. PMID: 39927512 Free PMC article.
-
Reconfigurable droplet networks.Nat Commun. 2024 Feb 5;15(1):1058. doi: 10.1038/s41467-024-45214-1. Nat Commun. 2024. PMID: 38316759 Free PMC article.
References
-
- Yang C, Suo Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018;3:125–142. doi: 10.1038/s41578-018-0018-7. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

