An orexigenic subnetwork within the human hippocampus
- PMID: 37648849
- PMCID: PMC10499606
- DOI: 10.1038/s41586-023-06459-w
An orexigenic subnetwork within the human hippocampus
Abstract
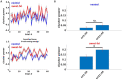
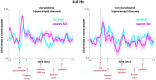
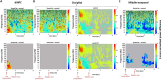
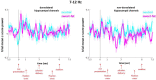
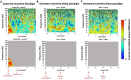
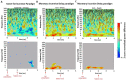
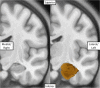
Only recently have more specific circuit-probing techniques become available to inform previous reports implicating the rodent hippocampus in orexigenic appetitive processing1-4. This function has been reported to be mediated at least in part by lateral hypothalamic inputs, including those involving orexigenic lateral hypothalamic neuropeptides, such as melanin-concentrating hormone5,6. This circuit, however, remains elusive in humans. Here we combine tractography, intracranial electrophysiology, cortico-subcortical evoked potentials, and brain-clearing 3D histology to identify an orexigenic circuit involving the lateral hypothalamus and converging in a hippocampal subregion. We found that low-frequency power is modulated by sweet-fat food cues, and this modulation was specific to the dorsolateral hippocampus. Structural and functional analyses of this circuit in a human cohort exhibiting dysregulated eating behaviour revealed connectivity that was inversely related to body mass index. Collectively, this multimodal approach describes an orexigenic subnetwork within the human hippocampus implicated in obesity and related eating disorders.
© 2023. The Author(s).
Conflict of interest statement
C.H.H. receives consulting and speaking honoraria from Boston Scientific and Insightec. C.H.H, D.A.N.B., S.G. and Y.H. have patents owned by Stanford University related to sensing and brain stimulation for the treatment of neuropsychiatric disorders: USPTO serial number: 63/170,404 and 63/220,432; international publication number: WO 2022/212891 A1 (international publication date: 6 October 2022). C.H.H., D.A.N.B. and J.A.M. have patents related to using tractography for circuit-based brain stimulation: USPTO serial number: 63/210,472; international publication number: WO 2022/266000 (international publication date: 22 December 2022). P.R. declares that the opinions and assertions contained in this manuscript do not necessarily reflect the official policy or position of the Uniformed Services University, the Department of Defense or the Henry M. Jackson Foundation for the Advancement of Military medicine. B.D.H. is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant for Clairvoyant Therapeutics and Vine Ventures. The other authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

