Hippocampal representation during collective spatial behaviour in bats
- PMID: 37648869
- PMCID: PMC10533399
- DOI: 10.1038/s41586-023-06478-7
Hippocampal representation during collective spatial behaviour in bats
Abstract
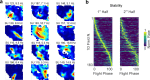
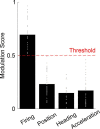
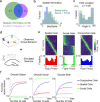
Social animals live and move through spaces shaped by the presence, motion and sensory cues of multiple other individuals1-6. Neural activity in the hippocampus is known to reflect spatial behaviour7-9 yet its study is lacking in such dynamic group settings, which are ubiquitous in natural environments. Here we studied hippocampal activity in groups of bats engaged in collective spatial behaviour. We find that, under spontaneous conditions, a robust spatial structure emerges at the group level whereby behaviour is anchored to specific locations, movement patterns and individual social preferences. Using wireless electrophysiological recordings from both stationary and flying bats, we find that many hippocampal neurons are tuned to key features of group dynamics. These include the presence or absence of a conspecific, but not typically of an object, at landing sites, shared spatial locations, individual identities and sensory signals that are broadcasted in the group setting. Finally, using wireless calcium imaging, we find that social responses are anatomically distributed and robustly represented at the population level. Combined, our findings reveal that hippocampal activity contains a rich representation of naturally emerging spatial behaviours in animal groups that could in turn support the complex feat of collective behaviour.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures















Similar articles
-
Neural representation of human experimenters in the bat hippocampus.Nat Neurosci. 2024 Sep;27(9):1675-1679. doi: 10.1038/s41593-024-01690-8. Epub 2024 Jul 2. Nat Neurosci. 2024. PMID: 38956164 Free PMC article.
-
A stable hippocampal code in freely flying bats.Nature. 2022 Apr;604(7904):98-103. doi: 10.1038/s41586-022-04560-0. Epub 2022 Mar 30. Nature. 2022. PMID: 35355012 Free PMC article.
-
Cortical representation of group social communication in bats.Science. 2021 Oct 22;374(6566):eaba9584. doi: 10.1126/science.aba9584. Epub 2021 Oct 22. Science. 2021. PMID: 34672724 Free PMC article.
-
Social communication in bats.Biol Rev Camb Philos Soc. 2018 Nov;93(4):1938-1954. doi: 10.1111/brv.12427. Epub 2018 May 15. Biol Rev Camb Philos Soc. 2018. PMID: 29766650 Review.
-
Do predators influence the behaviour of bats?Biol Rev Camb Philos Soc. 2013 Aug;88(3):626-44. doi: 10.1111/brv.12021. Epub 2013 Jan 24. Biol Rev Camb Philos Soc. 2013. PMID: 23347323 Review.
Cited by
-
Advertisement vocalizations support home-range defense in the singing mouse.Curr Biol. 2025 Jun 23;35(12):2960-2966.e5. doi: 10.1016/j.cub.2025.04.034. Epub 2025 May 7. Curr Biol. 2025. PMID: 40339572
-
Neural Processing without O2 and Glucose Delivery: Lessons from the Pond to the Clinic.Physiology (Bethesda). 2024 Nov 1;39(6):0. doi: 10.1152/physiol.00030.2023. Epub 2024 Apr 16. Physiology (Bethesda). 2024. PMID: 38624246 Review.
-
Neuronal activity underlying vocal production in bats.Ann N Y Acad Sci. 2025 Aug;1550(1):37-54. doi: 10.1111/nyas.15410. Epub 2025 Jul 21. Ann N Y Acad Sci. 2025. PMID: 40692182 Free PMC article. Review.
-
Neural representation of human experimenters in the bat hippocampus.Nat Neurosci. 2024 Sep;27(9):1675-1679. doi: 10.1038/s41593-024-01690-8. Epub 2024 Jul 2. Nat Neurosci. 2024. PMID: 38956164 Free PMC article.
-
The study of social animal migrations: a synthesis of the past and guidelines for future research.Proc Biol Sci. 2025 Jun;292(2049):20242726. doi: 10.1098/rspb.2024.2726. Epub 2025 Jun 18. Proc Biol Sci. 2025. PMID: 40527454 Free PMC article. Review.
References
-
- Couzin ID. Collective cognition in animal groups. Trends Cogn. Sci. 2009;13:36–43. - PubMed
-
- Egert-Berg K, et al. Resource ephemerality drives social foraging in bats. Curr. Biol. 2018;28:3667–3673. - PubMed
-
- Dibnah AJ, et al. Vocally mediated consensus decisions govern mass departures from jackdaw roosts. Curr. Biol. 2022;32:R455–R456. - PubMed

