Beyond the anti-PD-1/PD-L1 era: promising role of the BTLA/HVEM axis as a future target for cancer immunotherapy
- PMID: 37649037
- PMCID: PMC10466776
- DOI: 10.1186/s12943-023-01845-4
Beyond the anti-PD-1/PD-L1 era: promising role of the BTLA/HVEM axis as a future target for cancer immunotherapy
Abstract
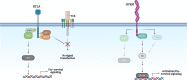
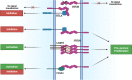
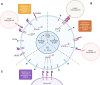
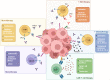
Recent introduction of monoclonal antibodies targeting immune checkpoints to harness antitumor immunity has revolutionized the cancer treatment landscape. The therapeutic success of immune checkpoint blockade (ICB)-based therapies mainly relies on PD-1/PD-L1 and CTLA-4 blockade. However, the limited overall responses and lack of reliable predictive biomarkers of patient´s response are major pitfalls limiting immunotherapy success. Hence, this reflects the compelling need of unveiling novel targets for immunotherapy that allow to expand the spectrum of ICB-based strategies to achieve optimal therapeutic efficacy and benefit for cancer patients. This review thoroughly dissects current molecular and functional knowledge of BTLA/HVEM axis and the future perspectives to become a target for cancer immunotherapy. BTLA/HVEM dysregulation is commonly found and linked to poor prognosis in solid and hematological malignancies. Moreover, circulating BTLA has been revealed as a blood-based predictive biomarker of immunotherapy response in various cancers. On this basis, BTLA/HVEM axis emerges as a novel promising target for cancer immunotherapy. This prompted rapid development and clinical testing of the anti-BTLA blocking antibody Tifcemalimab/icatolimab as the first BTLA-targeted therapy in various ongoing phase I clinical trials with encouraging results on preliminary efficacy and safety profile as monotherapy and combined with other anti-PD-1/PD-L1 therapies. Nevertheless, it is anticipated that the intricate signaling network constituted by BTLA/HVEM/CD160/LIGHT involved in immune response regulation, tumor development and tumor microenvironment could limit therapeutic success. Therefore, in-depth functional characterization in different cancer settings is highly recommended for adequate design and implementation of BTLA-targeted therapies to guarantee the best clinical outcomes to benefit cancer patients.
Keywords: BTLA; CD160; Checkpoint blockade; HVEM; Icatolimab; Immunotherapy; LIGHT; NK cell; T cell; Tifcemalimab.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
All authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

