A conserved protein of Babesia microti elicits partial protection against Babesia and Plasmodium infection
- PMID: 37649042
- PMCID: PMC10469411
- DOI: 10.1186/s13071-023-05825-x
A conserved protein of Babesia microti elicits partial protection against Babesia and Plasmodium infection
Abstract
Background: The protozoan parasite Babesia microti that causes the zoonotic disease babesiosis resides in the erythrocytes of its mammalian host during its life-cycle. No effective vaccines are currently available to prevent Babesia microti infections.
Methods: We previously identified a highly seroactive antigen, named Bm8, as a B. microti conserved erythrocyte membrane-associated antigen, by high-throughput protein chip screening. Bioinformatic and phylogenetic analysis showed that this membrane-associated protein is conserved among apicomplexan hemoprotozoa, such as members of genera Babesia, Plasmodium and Theileria. We obtained the recombinant protein Bm8 (rBm8) by prokaryotic expression and purification.
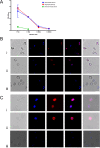
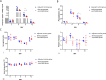
Results: Immunofluorescence assays confirmed that Bm8 and its Plasmodium homolog were principally localized in the cytoplasm of the parasite. rBm8 protein was specifically recognized by the sera of mice infected with B. microti or P. berghei. Also, mice immunized with Bm8 polypeptide had a decreased parasite burden after B. microti or P. berghei infection.
Conclusions: Passive immunization with Bm8 antisera could protect mice against B. microti or P. berghei infection to a certain extent. These results lead us to hypothesize that the B. microti conserved erythrocyte membrane-associated protein Bm8 could serve as a novel broad-spectrum parasite vaccine candidate since it elicits a protective immune response against Babesiosis and Plasmodium infection.
Keywords: Antigens; Babesia microti; Bioinformatic analysis; Conserved protein; Vaccine.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

