Biochemical characterisation of a novel broad pH spectrum subtilisin from Fictibacillus arsenicus DSM 15822T
- PMID: 37649135
- PMCID: PMC10626276
- DOI: 10.1002/2211-5463.13701
Biochemical characterisation of a novel broad pH spectrum subtilisin from Fictibacillus arsenicus DSM 15822T
Abstract
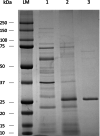
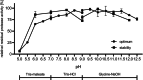
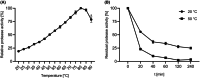
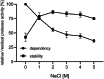
Subtilisins from microbial sources, especially from the Bacillaceae family, are of particular interest for biotechnological applications and serve the currently growing enzyme market as efficient and novel biocatalysts. Biotechnological applications include use in detergents, cosmetics, leather processing, wastewater treatment and pharmaceuticals. To identify a possible candidate for the enzyme market, here we cloned the gene of the subtilisin SPFA from Fictibacillus arsenicus DSM 15822T (obtained through a data mining-based search) and expressed it in Bacillus subtilis DB104. After production and purification, the protease showed a molecular mass of 27.57 kDa and a pI of 5.8. SPFA displayed hydrolytic activity at a temperature optimum of 80 °C and a very broad pH optimum between 8.5 and 11.5, with high activity up to pH 12.5. SPFA displayed no NaCl dependence but a high NaCl tolerance, with decreasing activity up to concentrations of 5 m NaCl. The stability enhanced with increasing NaCl concentration. Based on its substrate preference for 10 synthetic peptide 4-nitroanilide substrates with three or four amino acids and its phylogenetic classification, SPFA can be assigned to the subgroup of true subtilisins. Moreover, SPFA exhibited high tolerance to 5% (w/v) SDS and 5% H2 O2 (v/v). The biochemical properties of SPFA, especially its tolerance of remarkably high pH, SDS and H2 O2 , suggest it has potential for biotechnological applications.
Keywords: Bacillaceae; biotechnological application; broad pH spectrum; subtilases; subtilisin.
© 2023 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
New robust subtilisins from halotolerant and halophilic Bacillaceae.Appl Microbiol Biotechnol. 2023 Jun;107(12):3939-3954. doi: 10.1007/s00253-023-12553-w. Epub 2023 May 9. Appl Microbiol Biotechnol. 2023. PMID: 37160606 Free PMC article.
-
Biochemical characterization of a novel oxidatively stable, halotolerant, and high-alkaline subtilisin from Alkalihalobacillus okhensis Kh10-101T.FEBS Open Bio. 2022 Oct;12(10):1729-1746. doi: 10.1002/2211-5463.13457. Epub 2022 Jul 6. FEBS Open Bio. 2022. PMID: 35727859 Free PMC article.
-
AprMH1 subtilisin from Bacillus zhangzhouensis MH1: Molecular cloning, characterization, and homology modeling.Int J Biol Macromol. 2025 May;307(Pt 2):141889. doi: 10.1016/j.ijbiomac.2025.141889. Epub 2025 Mar 8. Int J Biol Macromol. 2025. PMID: 40064257
-
Fictibacillus phosphorivorans gen. nov., sp. nov. and proposal to reclassify Bacillus arsenicus, Bacillus barbaricus, Bacillus macauensis, Bacillus nanhaiensis, Bacillus rigui, Bacillus solisalsi and Bacillus gelatini in the genus Fictibacillus.Int J Syst Evol Microbiol. 2013 Aug;63(Pt 8):2934-2944. doi: 10.1099/ijs.0.049171-0. Epub 2013 Jan 25. Int J Syst Evol Microbiol. 2013. PMID: 23355698
-
Advances in protease engineering for laundry detergents.N Biotechnol. 2015 Dec 25;32(6):629-34. doi: 10.1016/j.nbt.2014.12.010. Epub 2015 Jan 8. N Biotechnol. 2015. PMID: 25579194 Review.
Cited by
-
Characterization, whole-genome sequence analysis, and protease production of a new thermophilic Bacillus licheniformis strain isolated from Debagh hot spring, Algeria.Int Microbiol. 2025 Apr;28(4):667-689. doi: 10.1007/s10123-024-00569-9. Epub 2024 Aug 12. Int Microbiol. 2025. PMID: 39129036
References
-
- Gupta R, Beg QK and Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59, 15–32. - PubMed
-
- Naveed M, Nadeem F, Mehmood T, Bilal M, Anwar Z and Amjad F (2021) Protease—a versatile and ecofriendly biocatalyst with multi‐industrial applications: an updated review. Catal Lett 151, 307–323.
-
- Saeki K, Magallones MV, Takimura Y, Hatada Y, Kobayashi T, Kawai S and Ito S (2003) Nucleotide and deduced amino acid sequences of a new subtilisin from an alkaliphilic Bacillus isolate. Curr Microbiol 47, 337–340. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

