Discovery of pyrimidine-tethered benzothiazole derivatives as novel anti-tubercular agents towards multi- and extensively drug resistant Mycobacterium tuberculosis
- PMID: 37649381
- PMCID: PMC10472891
- DOI: 10.1080/14756366.2023.2250575
Discovery of pyrimidine-tethered benzothiazole derivatives as novel anti-tubercular agents towards multi- and extensively drug resistant Mycobacterium tuberculosis
Abstract
In this study, new benzothiazole-pyrimidine hybrids (5a-c, 6, 7a-f, and 8-15) were designed and synthesised. Two different functionalities on the pyrimidine moiety of lead compound 4 were subjected to a variety of chemical changes with the goal of creating various functionalities and cyclisation to further elucidate the target structures. The potency of the new molecules was tested against different tuberculosis (TB) strains. The results indicated that compounds 5c, 5b, 12, and 15 (MIC = 0.24-0.98 µg/mL) are highly active against the first-line drug-sensitive strain of Mycobacterium tuberculosis (ATCC 25177). Thereafter, the anti-tubercular activity was evaluated against the two drug-resistant TB strains; ATCC 35822 and RCMB 2674, where, many compounds exhibited good activity with MIC = 0.98-62.5 3 µg/mL and 3.9-62.5 µg/mL, respectively. Compounds 5c and 15 having the highest anti-tubercular efficiency towards sensitive strain, displayed the best activity for the resistant strains by showing the MIC = 0.98 and 1.95 µg/mL for MDR TB, and showing the MIC = 3.9 and 7.81 µg/mL for XDR TB, consecutively. Finally, molecular docking studies were performed for the two most active compounds 5c and 15 to explore their enzymatic inhibitory activities.
Keywords: Benzothiazole; DprE1; anti-mycobacterial activity; molecular docking; screening.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

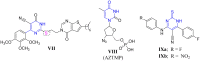







References
-
- Glaziou P, Floyd K, Raviglione MC.. Global epidemiology of tuberculosis. Semin Respir Crit Care Med. 2018;39(3):271–285. - PubMed
-
- Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Palomino JC.. Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2005;55(4):500–505. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
