A Biomimetic C-Terminal Extension Strategy for Photocaging Amidated Neuropeptides
- PMID: 37649440
- PMCID: PMC10510324
- DOI: 10.1021/jacs.3c03913
A Biomimetic C-Terminal Extension Strategy for Photocaging Amidated Neuropeptides
Abstract
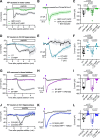
Photoactivatable neuropeptides offer a robust stimulus-response relationship that can drive mechanistic studies into the physiological mechanisms of neuropeptidergic transmission. The majority of neuropeptides contain a C-terminal amide, which offers a potentially general site for installation of a C-terminal caging group. Here, we report a biomimetic caging strategy in which the neuropeptide C-terminus is extended via a photocleavable amino acid to mimic the proneuropeptides found in large dense-core vesicles. We explored this approach with four prominent neuropeptides: gastrin-releasing peptide (GRP), oxytocin (OT), substance P (SP), and cholecystokinin (CCK). C-terminus extension greatly reduced the activity of all four peptides at heterologously expressed receptors. In cell type-specific electrophysiological recordings from acute brain slices, subsecond flashes of ultraviolet light produced rapidly activating membrane currents via activation of endogenous G protein-coupled receptors. Subsequent mechanistic studies with caged CCK revealed a role for extracellular proteases in shaping the temporal dynamics of CCK signaling, and a striking switch-like, cell-autonomous anti-opioid effect of transient CCK signaling in hippocampal parvalbumin interneurons. These results suggest that C-terminus extension with a photocleavable linker may be a general strategy for photocaging amidated neuropeptides and demonstrate how photocaged neuropeptides can provide mechanistic insights into neuropeptide signaling that are inaccessible using conventional approaches.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Smith S. J.; Smbül U.; Graybuck L. T.; Collman F.; Seshamani S.; Gala R.; Gliko O.; Elabbady L.; Miller J. A.; Bakken T. E.; Rossier J.; Yao Z.; Lein E.; Zeng H.; Tasic B.; Hawrylycz M. Single–Cell Transcriptomic Evidence for Dense Intracortical Neuropeptide Networks. Elife 2019, 8, e47889 10.7554/eLife.47889. - DOI - PMC - PubMed
-
- Xiong H.; Lacin E.; Ouyang H.; Naik A.; Xu X.; Xie C.; Youn J.; Wilson B. A.; Kumar K.; Kern T.; Aisenberg E.; Kircher D.; Li X.; Zasadzinski J. A.; Mateo C.; Kleinfeld D.; Hrabetova S.; Slesinger P. A.; Qin Z. Probing Neuropeptide Volume Transmission In Vivo by Simultaneous Near–Infrared Light-Triggered Release and Optical Sensing. Angew. Chem., Int. Ed. 2022, 61, e202206122 10.1002/anie.202206122. - DOI - PMC - PubMed

