Mechanoluminescence, thermoluminescence, optically stimulated luminescence and photoluminescence in SrAl2O4:Eu micro- and nanophosphors: effect of particle size and annealing in different atmospheres
- PMID: 37649574
- PMCID: PMC10463121
- DOI: 10.1039/d3ra02514d
Mechanoluminescence, thermoluminescence, optically stimulated luminescence and photoluminescence in SrAl2O4:Eu micro- and nanophosphors: effect of particle size and annealing in different atmospheres
Abstract
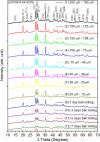
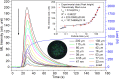
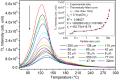
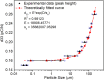
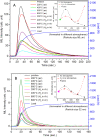
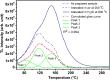
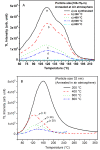
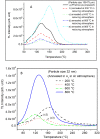
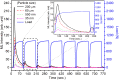
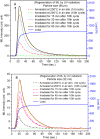
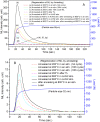
SrAl2O4:Eu in microcrystalline form was prepared by a combustion method. The formation of the material in a single phase was confirmed by XRD analysis. The material was crushed and sieved to get particles with different particle size ranges. It was further ball milled for 1-7 days to get particles in the nanosize ranges. The broadening of the XRD peaks of the phosphor material in nanocrystalline form was used to determine average particle sizes. The shapes and sizes of these particles could also be seen in FESEM images. The materials thus obtained were annealed in reducing (10% H2 in Ar) and oxidizing (in air) atmospheres at different temperatures for 1.0 h. The increase in the mechanoluminescence (ML) intensity on annealing in a reducing atmosphere at different temperatures and decrease on annealing in an oxidizing atmosphere could be attributed to redox reactions. This was further confirmed by PL measurements. Mechanoluminescence (ML), thermoluminescence (TL), and optically stimulated luminescence (OSL) of the materials were studied. In all three cases (i.e., ML, TL, and OSL), the intensities are found to decrease with the particle size. A large shift of approximately 20 °C in the main peak of TL glow curves of micro- and nanocrystalline materials shows a widening of the band gap due to the particle size effect. A decrease in piezoelectric constant (d33) and field (F V m-1) with particle size was also observed. The present systematic study of particle size effect (over a wide range of particle sizes) on ML has great importance from a technological and application point of view for developing stress sensors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest to declare.
Figures








Similar articles
-
Mechanoluminescence properties of SrAl2O4:Eu(2+) phosphor by combustion synthesis.Luminescence. 2016 Mar;31(2):394-400. doi: 10.1002/bio.2972. Epub 2015 Jul 21. Luminescence. 2016. PMID: 26198971
-
Synthesis, characterization and thermoluminescence studies of (ZnS)1-x (MnTe)x nanophosphors.Luminescence. 2017 May;32(3):375-381. doi: 10.1002/bio.3189. Epub 2016 Jul 28. Luminescence. 2017. PMID: 28394484
-
Thermoluminescence studies of CaSO4:Dy nanophosphor for application in high dose measurements.Appl Radiat Isot. 2019 Jun;148:253-261. doi: 10.1016/j.apradiso.2019.03.015. Epub 2019 Mar 12. Appl Radiat Isot. 2019. PMID: 31026790
-
Effect of size variation and gamma irradiation on thermoluminescence and photoluminescence characteristics of CaSO4:Eu micro- and nanophosphors.Appl Radiat Isot. 2020 May;159:109080. doi: 10.1016/j.apradiso.2020.109080. Epub 2020 Feb 26. Appl Radiat Isot. 2020. PMID: 32250756
-
Study of optically stimulated luminescence and calculation of trapping parameters of K2Ca2(SO4)3:Eu nanophosphor.Appl Radiat Isot. 2021 Jan;167:109388. doi: 10.1016/j.apradiso.2020.109388. Epub 2020 Sep 19. Appl Radiat Isot. 2021. PMID: 33011514
Cited by
-
Rare Earths-Doped and Ceria-Coated Strontium Aluminate PlateletsVersatile Luminescent Platforms for Correlated Lifetime Imaging by Multiphoton FLIM and PLIM.ACS Omega. 2025 Apr 29;10(19):19950-19965. doi: 10.1021/acsomega.5c01649. eCollection 2025 May 20. ACS Omega. 2025. PMID: 40415853 Free PMC article.
-
Recovering mechanoluminescence in SAOED/epoxy stress sensors with self-healing epoxy vitrimers.RSC Adv. 2025 Mar 6;15(9):7160-7171. doi: 10.1039/d4ra08911a. eCollection 2025 Feb 26. RSC Adv. 2025. PMID: 40052106 Free PMC article.
-
Unveiling Hidden Prints: Optically stimulated luminescence for latent fingerprint detection.Heliyon. 2023 Nov 23;9(12):e22794. doi: 10.1016/j.heliyon.2023.e22794. eCollection 2023 Dec. Heliyon. 2023. PMID: 38058441 Free PMC article.
References
-
- Chandra B. P., Mechanoluminescence, in Luminescence of Solids, ed. D. R. Vij, Plenum Press, New York, 1998, pp. 361-389
-
- Chandra B. P., Mechanoluminescent smart materials and their applications, in Electronic and Catalytic Properties of Advanced Materials, ed. A. Stashans, S. Gonzalez and H. P. Pinto, Transworld Research Network, Trivandrum, Kerala, India, 2011, pp. 1–37
-
- Xin P. Zhuang Y. Mei L. Xie R. J. Chin. Ceram. Soc. 2022;50:3147–3164.
-
- Xu C. N., Encyclopedia of Smart Materials, 2002, 1, pp. 190–201
-
- Feng A. Smet P. F. Materials. 2018;11:484. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

