Lymph node-targeting adjuvant/neoantigen-codelivering vaccines for combination glioblastoma radioimmunotherapy
- PMID: 37649594
- PMCID: PMC10465217
- DOI: 10.7150/thno.84443
Lymph node-targeting adjuvant/neoantigen-codelivering vaccines for combination glioblastoma radioimmunotherapy
Abstract
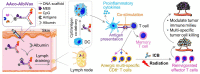
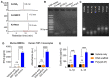
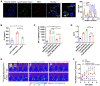
Glioblastoma multiforme (GBM) is the most common and lethal type of adult brain cancer. Current GBM standard of care, including radiotherapy, often ends up with cancer recurrence, resulting in limited long-term survival benefits for GBM patients. Immunotherapy, such as immune checkpoint blockade (ICB), has thus far shown limited clinical benefit for GBM patients. Therapeutic vaccines hold great potential to elicit anti-cancer adaptive immunity, which can be synergistically combined with ICB and radiotherapy. Peptide vaccines are attractive for their ease of manufacturing and stability, but their therapeutic efficacy has been limited due to poor vaccine co-delivery and the limited ability of monovalent antigen vaccines to prevent tumor immune evasion. To address these challenges, here, we report GBM radioimmunotherapy that combines radiotherapy, ICB, and multivalent lymph-node-targeting adjuvant/antigen-codelivering albumin-binding vaccines (AAco-AlbiVax). Specifically, to codeliver peptide neoantigens and adjuvant CpG to lymph nodes (LNs), we developed AAco-AlbiVax based on a Y-shaped DNA scaffold that was site-specifically conjugated with CpG, peptide neoantigens, and albumin-binding maleimide-modified Evans blue derivative (MEB). As a result, these vaccines elicited antitumor immunity including neoantigen-specific CD8+ T cell responses in mice. In orthotopic GBM mice, the combination of AAco-AlbiVax, ICB, and fractionated radiation enhanced GBM therapeutic efficacy. However, radioimmunotherapy only trended more efficacious over radiotherapy alone. Taken together, these studies underscore the great potential of radioimmunotherapy for GBM, and future optimization of treatment dosing and scheduling would improve the therapeutic efficacy.
Keywords: DNA engineering; albumin; glioblastoma immunotherapy; neoantigen vaccine; vaccine codelivery.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Ionizable polymeric nanocarriers for the codelivery of bi-adjuvant and neoantigens in combination tumor immunotherapy.Bioact Mater. 2023 Aug;26:169-180. doi: 10.1016/j.bioactmat.2023.02.016. Epub 2023 Mar 3. Bioact Mater. 2023. PMID: 36883121 Free PMC article.
-
Responsive Multivesicular Polymeric Nanovaccines that Codeliver STING Agonists and Neoantigens for Combination Tumor Immunotherapy.Adv Sci (Weinh). 2022 Aug;9(23):e2201895. doi: 10.1002/advs.202201895. Epub 2022 Jun 16. Adv Sci (Weinh). 2022. PMID: 35712773 Free PMC article.
-
Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy.Nat Commun. 2017 Dec 5;8(1):1954. doi: 10.1038/s41467-017-02191-y. Nat Commun. 2017. PMID: 29203865 Free PMC article.
-
Combination immunotherapy strategies for glioblastoma.J Neurooncol. 2021 Feb;151(3):375-391. doi: 10.1007/s11060-020-03481-0. Epub 2021 Feb 21. J Neurooncol. 2021. PMID: 33611705 Review.
-
Neoantigen discovery and applications in glioblastoma: An immunotherapy perspective.Cancer Lett. 2022 Dec 1;550:215945. doi: 10.1016/j.canlet.2022.215945. Epub 2022 Oct 7. Cancer Lett. 2022. PMID: 36216148 Review.
References
-
- Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu Rev Immunol. 2020;38:597–620. - PubMed
-
- Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2021;151:41–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

