Restoration of CPT1A-mediated fatty acid oxidation in mesothelial cells protects against peritoneal fibrosis
- PMID: 37649600
- PMCID: PMC10465225
- DOI: 10.7150/thno.84921
Restoration of CPT1A-mediated fatty acid oxidation in mesothelial cells protects against peritoneal fibrosis
Abstract
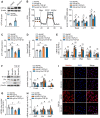
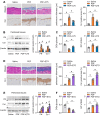
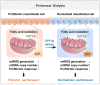
Background: Peritoneal dialysis (PD) is limited by gradual fibrotic remodeling in the peritoneum, a process involving profibrotic response of mesothelial cells. However, the role of fatty acid oxidation (FAO) and carnitine palmitoyltransferase 1A (CPT1A) in this process remains unexplored. Methods: FAO and CPT1A expression were characterized in mesothelial cells from patients on long-term PD and from a mouse model of PD using multiple experimental methods, including single-cell sequencing, seahorse assay, real-time quantitative PCR, Western blot, and immunofluorescence staining. Overexpression of CPT1A was achieved in a human mesothelial cell line and in primary mouse mesothelial cells. Finally, genetic and pharmacological manipulations of CPT1A were performed in a mouse model of PD. Results: Herein, FAO and CPT1A expression were reduced in mesothelial cells from patients on long-term PD, which negatively correlated with expression of fibrogenic markers in these cells. This was corroborated in PD mice, as well as in mouse and human mesothelial cells incubated with transforming growth factor (TGF) β1. CPT1A overexpression in mesothelial cells, which prevented TGFβ1-induced suppression of mitochondrial respiration, restored cellular ATP levels and downregulated the expression of fibrogenic markers. Furthermore, restoration of FAO by overexpressing CPT1A in PD mice reversed profibrotic phenotype in mesothelial cells and reduced fibrotic lesions in the peritoneum. Treatment with the CPT1A activator C75 induced similar therapeutic benefit in PD mice. In contrast, inhibition of FAO with a CPT1 inhibitor caused more severe fibrosis in PD mice. Conclusions: A defective FAO is responsible for the profibrotic response of mesothelial cells and thus the peritoneal fibrogenesis. This aberrant metabolic state could be improved by modulating CPT1A in mesothelial cells, suggesting FAO enhancement in mesothelial cells is a potential treatment of peritoneal fibrosis.
Keywords: carnitine palmitoyltransferase 1A; fatty acid oxidation; mesothelial cell; peritoneal dialysis; peritoneal fibrosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Inhibition of carnitine palmitoyltransferase 1A in hepatic stellate cells protects against fibrosis.J Hepatol. 2022 Jul;77(1):15-28. doi: 10.1016/j.jhep.2022.02.003. Epub 2022 Feb 12. J Hepatol. 2022. PMID: 35167910
-
Renal tubule Cpt1a overexpression protects from kidney fibrosis by restoring mitochondrial homeostasis.J Clin Invest. 2021 Mar 1;131(5):e140695. doi: 10.1172/JCI140695. J Clin Invest. 2021. PMID: 33465052 Free PMC article.
-
Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer.Oncotarget. 2016 Jan 26;7(4):3832-46. doi: 10.18632/oncotarget.6757. Oncotarget. 2016. PMID: 26716645 Free PMC article.
-
CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential.Endocrinology. 2020 Feb 1;161(2):bqz046. doi: 10.1210/endocr/bqz046. Endocrinology. 2020. PMID: 31900483 Review.
-
A promising anti-tumor targeting on ERMMDs mediated abnormal lipid metabolism in tumor cells.Cell Death Dis. 2024 Aug 4;15(8):562. doi: 10.1038/s41419-024-06956-4. Cell Death Dis. 2024. PMID: 39098929 Free PMC article. Review.
Cited by
-
Peritoneal Dialysis -Associated Fibrosis: Emerging Mechanisms and Therapeutic Opportunities.Front Pharmacol. 2025 Aug 22;16:1635624. doi: 10.3389/fphar.2025.1635624. eCollection 2025. Front Pharmacol. 2025. PMID: 40918510 Free PMC article. Review.
-
The role of lipid metabolism imbalance in copper-induced PANoptosis in broiler kidney.Poult Sci. 2025 Jul 15;104(10):105549. doi: 10.1016/j.psj.2025.105549. Online ahead of print. Poult Sci. 2025. PMID: 40669240 Free PMC article.
-
Intercellular communication in peritoneal dialysis.Front Physiol. 2024 Feb 8;15:1331976. doi: 10.3389/fphys.2024.1331976. eCollection 2024. Front Physiol. 2024. PMID: 38390449 Free PMC article. Review.
-
CPT1A mediated preservation of mitochondrial inhibits pyroptosis in pancreatic acinar cells.Front Cell Dev Biol. 2025 Jul 11;13:1577669. doi: 10.3389/fcell.2025.1577669. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40718711 Free PMC article.
-
Inhibition of adenosine/A2A receptor signaling suppresses dermal fibrosis by enhancing fatty acid oxidation.Cell Commun Signal. 2025 Apr 29;23(1):206. doi: 10.1186/s12964-025-02210-2. Cell Commun Signal. 2025. PMID: 40301970 Free PMC article.
References
-
- Cho Y, Bello AK, Levin A, Lunney M, Osman MA, Ye F. et al. Peritoneal Dialysis Use and Practice Patterns: An International Survey Study. Am J Kidney Dis. 2021;77:315–25. - PubMed
-
- Teitelbaum I. Peritoneal Dialysis. N Engl J Med. 2021;385:1786–95. - PubMed
-
- Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol. 2006;17:2591–8. - PubMed
-
- Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol. 2010;21:1077–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

