Oleic acid-induced metastasis of KRAS/p53-mutant colorectal cancer relies on concurrent KRAS activation and IL-8 expression bypassing EGFR activation
- PMID: 37649607
- PMCID: PMC10465226
- DOI: 10.7150/thno.85855
Oleic acid-induced metastasis of KRAS/p53-mutant colorectal cancer relies on concurrent KRAS activation and IL-8 expression bypassing EGFR activation
Abstract
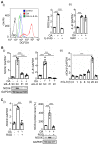
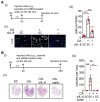
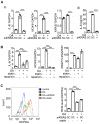
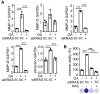
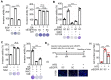
Background: Multigene mutations in colorectal cancer (CRC), including KRAS, BRAF, and p53, afford high metastatic ability and resistance to EGFR-targeting therapy. Understanding the molecular mechanisms regulating anti-EGFR-resistant CRC metastasis can improve CRC therapy. This study aimed to investigate the effects of IL-8 and the activation of KRAS on reactive oxygen species (ROS) production and metastasis of hyperlipidemia-associated CRC harboring mutations of KRAS and p53. Methods: The cytokine array analysis determined the up-expression of secreted factors, including IL-8. The clinical relevance of the relationship between IL-8 and angiopoietin-like 4 (ANGPTL4) was examined in CRC patients from National Cheng Kung University Hospital and TCGA dataset. Expressions of IL-8, ANGPTL4, NADPH oxidase 4 (NOX4), and epithelial-mesenchymal transition (EMT) markers in free fatty acids (FFAs)-treated KRAS/p53 mutant CRC cells were determined. The hyperlipidemia-triggered metastatic ability of CRC cells under treatments of antioxidants, statin, and cetuximab or knockdown of IL-8, KRAS, and EGFR was evaluated in vitro and in vivo. In addition, the effects of antioxidants and depletion of IL-8 and KRAS on the correlation between ROS production and hyperlipidemia-promoted CRC metastasis were also clarified. Results: In this study, we found that free fatty acids promoted KRAS/p53-mutant but not single-mutant or non-mutant CRC cell metastasis. IL-8, the most abundant secreted factor in KRAS/p53-mutant cells, was correlated with the upregulation of NOX4 expression and ROS production under oleic acid (OA)-treated conditions. In addition, the metastasis of KRAS/p53-mutant CRC relies on the ANGPTL4/IL-8/NOX4 axis and the activation of KRAS. The antioxidants and inactivation of KRAS also inhibited OA-induced EMT and metastasis. Although KRAS mediated EGF- and OA-promoted CRC cell invasion, the inhibition of EGFR did not affect OA-induced ANGPTL4/IL-8/NOX4 axis and CRC metastasis. The high-fat diet mice fed with vitamin E and statin or in IL-8-depleted cells significantly inhibited tumor extravasation and metastatic lung growth of CRC. Conclusion: The antioxidants, statins, and targeting IL-8 may provide better outcomes for treating metastatic CRC that harbors multigene mutations and anti-EGFR resistance.
Keywords: IL-8; KRAS activation; metastasis; multigene mutant CRC; oleic acid.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A. et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30:653–60. - PubMed
-
- Dueland S, Guren TK, Hagness M, Glimelius B, Line P-D, Pfeiffer P. et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg. 2015;261:956–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

