Effect of endophytic diazotroph Enterobacter roggenkampii ED5 on nitrogen-metabolism-related microecology in the sugarcane rhizosphere at different nitrogen levels
- PMID: 37649627
- PMCID: PMC10464614
- DOI: 10.3389/fmicb.2023.1132016
Effect of endophytic diazotroph Enterobacter roggenkampii ED5 on nitrogen-metabolism-related microecology in the sugarcane rhizosphere at different nitrogen levels
Erratum in
-
Corrigendum: Effect of endophytic diazotroph Enterobacter roggenkampii ED5 on nitrogen-metabolism-related microecology in the sugarcane rhizosphere at different nitrogen levels.Front Microbiol. 2023 Sep 27;14:1290575. doi: 10.3389/fmicb.2023.1290575. eCollection 2023. Front Microbiol. 2023. PMID: 37829447 Free PMC article.
Abstract
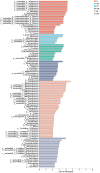
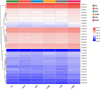
Sugarcane is an important sugar and energy crop worldwide, requiring a large amount of nitrogen (N). However, excessive application of synthetic N fertilizer causes environmental pollution in farmland. Endophytic nitrogen-fixing bacteria (ENFB) provide N nutrition for plants through biological N fixation, thus reducing the need for chemical fertilizers. The present study investigated the effect of the N-fixing endophytic strain Enterobacter roggenkampii ED5 on phytohormone indole-3-acetic acid (IAA), N-metabolism enzyme activities, microbial community compositions, and N cycle genes in sugarcane rhizosphere soil at different N levels. Three levels of 15N-urea, such as low N (0 kg/ha), medium N (150 kg/ha), and high N (300 kg/ha), were applied. The results showed that, after inoculating strain ED5, the IAA content in sugarcane leaves was significantly increased by 68.82% under low N condition at the seedling stage (60 days). The nitrate reductase (NR) activity showed a downward trend. However, the glutamine synthase (GS) and NADH-glutamate dehydrogenase (NADH-GDH) activities were significantly enhanced compared to the control under the high N condition, and the GS and NR genes had the highest expression at 180 and 120 days, respectively, at the low N level. The total N content in the roots, stems, and leaves of sugarcane was higher than the control. The 15N atom % excess of sugarcane decreased significantly under medium N condition, indicating that the medium N level was conducive to N fixation in strain ED5. Metagenome analysis of sugarcane rhizosphere soil exhibited that the abundance of N-metabolizing microbial richness was increased under low and high N conditions after inoculation of strain ED5 at the genus level, while it was increased at the phylum level only under the low N condition. The LefSe (LDA > 2, p < 0.05) found that the N-metabolism-related differential microorganisms under the high N condition were higher than those under medium and low N conditions. It was also shown that the abundance of nifDHK genes was significantly increased after inoculation of ED5 at the medium N level, and other N cycle genes had high abundance at the high N level after inoculation of strain ED5. The results of this study provided a scientific reference for N fertilization in actual sugarcane production.
Keywords: Enterobacter roggenkampii ED5; metagenomics; nitrogen fixation; nitrogen levels; rhizosphere; sugarcane growth.
Copyright © 2023 Guo, Li, Chen, Yang, Verma, Singh, Singh, Khan, Sharma, Qin, Zhang, Song and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Alotaibi M. O., Alotibi M. M., Eissa M. A., Ghoneim A. M. (2022). Compost and plant growth-promoting bacteria enhanced steviol glycoside synthesis in stevia (Stevia rebaudiana Bertoni) plants by improving soil quality and regulating nitrogen uptake. S. Afr. J. Bot. 151, 306–314. 10.1016/j.sajb.2022.10.010 - DOI
-
- Aquino J. P. A., Antunes J. E. L., Bonifácio A., Rocha S. M. B., Amorim M. R., Alcântara Neto F., et al. (2021). Plant growth-promoting bacteria improve growth and nitrogen metabolism in maize and sorghum. Theor. Exp. Plant phys. 33, 249–260. 10.1007/s40626-021-00209-x - DOI
LinkOut - more resources
Full Text Sources

