Impact of the carbon flux regulator protein pirC on ethanol production in engineered cyanobacteria
- PMID: 37649635
- PMCID: PMC10465007
- DOI: 10.3389/fmicb.2023.1238737
Impact of the carbon flux regulator protein pirC on ethanol production in engineered cyanobacteria
Abstract
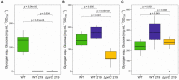
Future sustainable energy production can be achieved using mass cultures of photoautotrophic microorganisms such as cyanobacteria, which are engineered to synthesize valuable products directly from CO2 and sunlight. For example, strains of the model organism Synechocystis sp. PCC 6803 have been generated to produce ethanol. Here, we performed a study to prove the hypothesis that carbon flux in the direction of pyruvate is one bottleneck to achieve high ethanol titers in cyanobacteria. Ethanol-producing strains of the cyanobacterium Synechocystis sp. PCC 6803 were generated that bear mutation in the gene pirC aiming to increase carbon flux towards pyruvate. The strains were cultivated at different nitrogen or carbon conditions and the ethanol production was analysed. Generally, a clear correlation between growth rate and ethanol production was found. The mutation of pirC, however, had only a positive impact on ethanol titers under nitrogen depletion. The increase in ethanol was accompanied by elevated pyruvate and lowered glycogen levels indicating that the absence of pirC indeed increased carbon partitioning towards lower glycolysis. Metabolome analysis revealed that this change in carbon flow had also a marked impact on the overall primary metabolism in Synechocystis sp. PCC 6803. Deletion of pirC improved ethanol production under specific conditions supporting the notion that a better understanding of regulatory mechanisms involved in cyanobacterial carbon partitioning is needed to engineer more productive cyanobacterial strains.
Keywords: CO2; biofuel; carbon availability; green biotechnology; nitrogen limitation.
Copyright © 2023 Böhm, Kauss, Michl, Engelhardt, Brouwer and Hagemann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
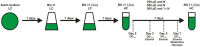




References
-
- Dexter J., Fu P. (2009). Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2, 857–864. doi: 10.1039/B811937F - DOI
LinkOut - more resources
Full Text Sources

